
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLet us see how bases react with metal with the help of some activities:
Important!
Caution: The experiment should be performed in the presence of a teacher.
Example:
Activity 1: The experiment aims to see how do bases react with metals.
Apparatus required:
- Test tube
- Delivery tube
- Glass tub
- Candle
- Zinc granules
- Water
- Soap
- Sodium hydroxide NaOH
Step 1: Take a test tube and add 2mL of sodium hydroxide (NaOH\) solution and add a few pinches of zinc granules.
Step 2: Heat the test tube for few minutes until it is warm.
Step 3:
- Observe the test tube for any changes.
- Fit the test tube mouth with a cork.
- Attach one end of the delivery tube to it.
Step 4: The other end of the delivery tube should be placed inside the glass tube filled with a soap solution.
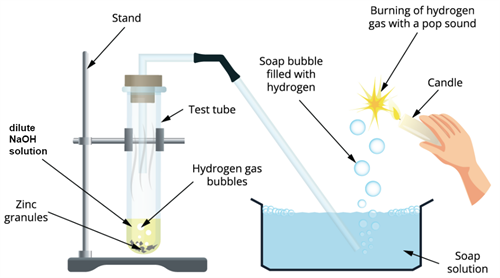
Bases on reaction with metals
Observation: The liberation of gas from the reaction passes through the soap tub forming bubbles.
Result: Since the hydrogen gas does not dissolve in a soap solution, it forms hydrogen gas bubbles
To prove that the formed gas is hydrogen, we can do a small test using a candle.
Take a burning candle near a gas-filled bubble that is formed from the above reaction. We can find the burning of hydrogen gas with a pop sound and turns the candle off.
Final observation:
- A colourless and odourless gas is formed.
- The formed gas does not dissolve in a soap solution, as a result bubbles are formed.
- The liberated gas found to be as hydrogen, which produces a pop sound and turns off the candle.
Conclusion:
- Sodium hydroxide + Zinc\rightarrow Salt + Hydrogen gas
- Therefore, the chemical reaction can be written as 2NaOH + Zn\rightarrow Na_2ZnO_2+ H_2
Here the formed salt is sodium zincate with the chemical formula Na_2ZnO_2.
Therefore, the overall reaction can be written as
Base+ Metal \rightarrow Salt + Hydrogen gas
We know that a chemical reaction occurs between metal and a base. But, it is not possible with all metals as most of the metals have the same characteristics as that of a base.
Note: If the cation of the base is less reactive than the metal, the reaction is possible.