PDF chapter test TRY NOW
How do you know if a chemical reaction has taken place or not?
When a chemical reaction occurs, there is a change in-
-Colour
-State
-Temperature
-Evolution of gas
Example:
Activity 1.1: The experiment aims to see how the state will change (burning magnesium ribbon in the air).
Important!
The experiment should proceed only in the presence of a teacher. If possible, students should wear eye protection (safety glasses).
Materials required:
-Magnesium ribbon (2 to 3 cm long)
-Two tongs
-Burner
-Two goggles
-Watch-glass
-Red and blue litmus papers
-Distilled water
-Beaker and
-Sandpaper
-Two tongs
-Burner
-Two goggles
-Watch-glass
-Red and blue litmus papers
-Distilled water
-Beaker and
-Sandpaper
Experimental procedure:
Step 1: Take a magnesium ribbon (2 to 3 cm long) and clean it with sandpaper. This cleaning will remove the oxide layer placed on the magnesium ribbon, which makes it inactive.
Step 2: Hold the magnesium ribbon over a watch glass with tongs and burn it in the air with a burner (as you see in the figure). Using a pair of dark-coloured goggles, watch the burning magnesium ribbon.
Step 3: Take a watch glass and collect the white powder ashes (magnesium ribbon).
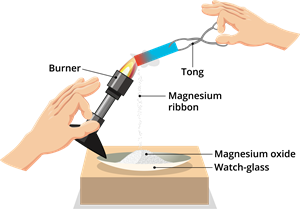
Magnesium ribbon is burned in the air, as magnesium oxide is collected in a watch-glass
Observation:
- We observe that the magnesium ribbon (reactant) burned with a dazzling white flame.
- The ribbon is converted into a (product) white powder.
- It is produced due to the reaction between magnesium and oxygen (air).
Result:
The white powder collected after burning the magnesium ribbon is magnesium oxide (MgO).
2Mg + O_2 → 2MgO
(magnesium ribbon + oxygen (air) → magnesium oxide)
Reactants → Products
We can do a small test using litmus paper to prove that the formed substance (magnesium oxide) is acid or base.
Step 1: Transfer the white powder to a beaker with a little distilled water and mix it well.
Step 2: Place a few drops of this mixture onto the red and blue litmus papers and record your observations.
Step 2: Place a few drops of this mixture onto the red and blue litmus papers and record your observations.
Final observation:
On placing a drop of the mixture over the red litmus paper, the colour of red litmus paper changes into blue.
On placing a drop of the mixture over the blue litmus paper, the colour of blue litmus paper remains the same (no change).
The difference in the colour of red litmus paper into blue suggests that the aqueous solution of magnesium oxide is basic in nature.
or
An unchanged colour of blue litmus paper suggests that the aqueous solution of magnesium oxide is basic in nature.
or
An unchanged colour of blue litmus paper suggests that the aqueous solution of magnesium oxide is basic in nature.
MgO(s) + H_2O(l) → Mg(OH)_2(aq)
Here the reaction is:
O_2(s) + H_2O(l) → 2OH(aq)
Here the reaction is:
O_2(s) + H_2O(l) → 2OH(aq)
We already know that a change in state is also a chemical reaction. This activity clearly shows the occurrence of the chemical reaction (state change), which means the conversion of ribbon (magnesium) into powder (magnesium oxide). Furthermore, the magnesium oxide (MgO) dissolves in water to form magnesium hydroxide (Mg(OH)_2, which acts as a strong base and turns red litmus paper into blue or does not change the blue litmus paper.
Example:
