
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe have learned in previous class that atoms of one element do not change into those of another element during a chemical reaction., Nor do atoms disappear from the mixture or appear from elsewhere. Chemical reactions give rise to new substances that form and break bonds between atoms. You will study about types of bonds formed between atoms in (carbon and its compounds and periodic classification of elements)
Chemical reactions can be divided into different classes. This classification is based on the specific properties and the nature of the reactions. The chemical reactions have been divided into the following types. These are
- Combination
- Decomposition
- Displacement
- Double displacement and
- Oxidation and reduction
Combination reaction:
A combination reaction is a process in which two or more substances are combined to form a single substance. Or Two reactants are combined to make a single compound.
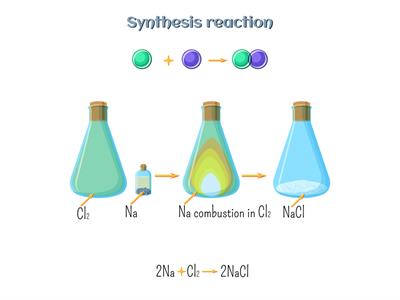
Combination reaction example
Activity 1.4: The goal of the experiment is to see how the combination reaction will take place.
Materials required:
-Calcium oxide
-Beaker and
-Water
Experimental procedure:
Step 1: Take some calcium oxide.
Step 2: Slowly add water to this calcium oxide.
Step 3: Slacked lime is formed, releasing a large amount of heat.
Observation:
- Hissy noise was produced
- Exothermic reaction takes place
- Water starts boiling
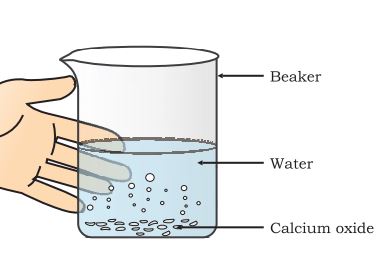
Combination reaction: calcium hydroxide formation
The beaker will become hot from the outside because of the liberation of a large amount of heat.
Result:
Calcium oxide combines vigorously with water to produce calcium hydroxide (slacked lime), releasing a large amount of heat.
CaO(s) + H_2O(l) → Ca(OH)_2(aq)
Quick lime Slacked lime
Conclusion:
In this activity, calcium oxide and water combine to form calcium hydroxide, a single product. This reaction (two or more reactants combined to form a single product) is a combination reaction.
CaO + H_2O → Ca(OH)_2 (calcium hydroxide)
lime lime water (colourless)
A solution of slaked lime formed by the above reaction is used for whitewashing walls.
Calcium hydroxide reacts gently with the carbon dioxide in the air to produce a thin layer of calcium carbonate on the walls.
Ca(OH)_2(aq) + CO_2(g) → CaCO_3(s) + H_2O(l)
(calcium hydroxide) (calcium carbonate, a white precipitate)
After two to three days of whitewashing, calcium carbonate is formed, giving the walls a lustrous sheen. It is worth noting that marble has the same chemical formula as calcium carbonate (CaCO_3).