
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe have already learnt that when metals react with acids, they form salt and hydrogen gas.
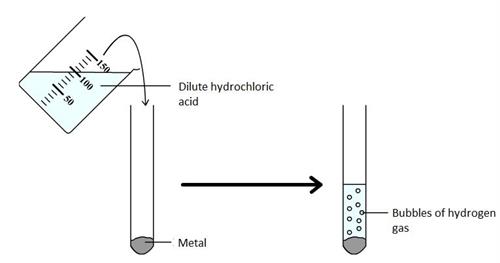
The reaction of a metal with hydrochloric acid
Example:
Here, (s), (aq) and (g) refer solid, aqueous and gaseous state, respectively.
In case of copper, no bubbles will appear and the temperature will remain unchanged. This proves that copper does not react with dilute HCl.
There is no hydrogen gas released when a metal reacts with nitric acid. It is because HNO_3 is a powerful oxidising agent. By oxidising the H_2 generated to water, it reduces itself to any of the nitrogen oxides (N_2O, NO, NO_2).
Furthermore, magnesium (Mg) and manganese (Mn) react with very dilute HNO_3 to produce H_2 gas. The reaction was also the highlyexothermic in this case. The reactivity decreases in the order Mg > Al > Zn > Fe.
Metals such as gold and silver are known to be unreactive to both HCl and HNO_3. Furthermore, the combination of these two acids form aquaregia, which can dissolve gold. It is a combination of hydrochloric acid and nitric acid mixed in the ratio of 3:1 respectively. It is a fuming yellow-orange that is highly corrosive. The chemical formula of aquaregia is 3HCl+HNO_3.
Reference:
http://www.physics-chemistry-class.com/reaction-iron-hydrocloric-acid.jpg