
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoSilver has a wide variety of used in everyday life and used in silverware and jewellery. When silver objects are exposed to air for an extended period of time, they turn black. This occurs when silver combines with sulphur in the air to form a silver sulphide coating.

Corroded silver jewellery
Similarly when copper reacts with moist carbon dioxide in the air, it loses its shiny brown surface and develops a green coating. This green substance is copper carbonate.
Formation of rust:
Iron is one of the most commonly used metal and it has a wide variety of applications for humans. However when iron is exposed to wet air for an extended period of time, it corrodes forming a coating called rust which is a brown flaky substance.
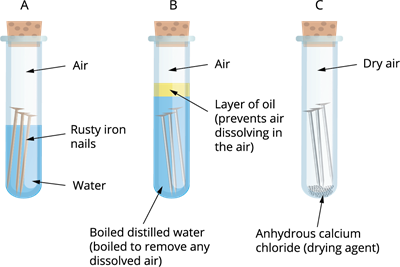
The conditions under which iron rusts are being investigated. Both air and water are present in tube A. There is no air dissolved in the water in tube B. The air in tube C is dry.
Iron nails rust in test tube A, but not in test tubes B or C, as you will observe. The nails in test tube A are both air and water exposure. The nails in test tube B are only exposed to water, whereas those in test tube C are only exposed to dry air.
Prevention of corrosion
- Painting, oiling, greasing, galvanising, chrome plating, anodizing, and alloying can all be used to keep iron against rusting.
- Galvanisation is a process that coats steel and iron with a thin layer of zinc to prevent rusting. Even if the zinc covering on the galvanised item is broken, it remains protected against rusting.
- Alloying is an excellent way to improve a metal's properties. This method can be used to obtain the desired properties. For example, iron is the most commonly used metal. However, it is never used in its pure state. This is due to the fact that pure iron is extremely soft and stretches easily when heated. It becomes hard and strong when mixed with a small amount of carbon (approximately 0.05 percent).
- When iron is combined with nickel and chromium, stainless steel is formed, which is hard and rust-resistant.
- When iron is combined with another substance, its properties are changed. In fact, any metal's properties can be altered by mixing it with another substance. It does not matter if the substance is metal or non-metal. A homogenous mixture of two or more metals, or a metal and a nonmetal, is known as an alloy. It's prepared by melting the primary metal first, then dissolving the other elements in it in definite proportions. After that, it's cooled to room temperature.
Do you know?
Pure gold, generally known as 24-carat gold, is a soft metal. As a result, it cannot be used to make jewellery. To make it harder, it is alloyed with silver or copper. In India, 22-carat gold is commonly used for jewellery. It involves 22 parts pure gold and 2 parts copper or silver alloyed together.
The wonder of ancient Indian metallurgy:
India's ironworkers created an iron pillar near the Qutub Minar in Delhi, approximately 400 BC. They had invented a method for preventing wrought the iron from rusting. This is most likely due to the formation of a thin film of magnetic oxide (Fe_3O_4) on the surface due to the pillar's finishing treatment, which included painting it with a mixture of salts, heating, and quenching. The iron pillar stands 8 metres tall and weighs 6 tonnes (6000 kg).
Reference:
https://th.bing.com/th/id/OIP.2q8wHQBtT6cTiWp7Lx6F6AAAAA?pid=ImgDet&rs=1
https://live.staticflickr.com/6091/6257265455_aed62037cc_b.jpg