PDF chapter test TRY NOW
The technique used to apply a metal to another metal is called electroplating.
In a brand new bicycle, the handlebar looks like steel with shining. This is due to the coating of some other metal. If the bicycle gets scratched, the shining will disappear, and it reveals the other metal beneath.
Similarly, women use ornaments that give a gold look. However, with repeated use, the gold coating will disappear and revealing some other metal look.
Chromium plating is applied on different objects like cars.



These can be achieved by the electroplating process.
Electroplating of a brass plate by copper:
- We need a brass plate and a copper plate.
- Rub the brass plate with sandpaper, wash and dry.
- The more the rubbed surface, the more will be the chemical reaction and result will get quickly.
- Wash a beaker and dry.
- Make a copper sulphate solution by adding two teaspoons of copper sulphates (CuSO_4) and dilute sulphuric acid into 250 mL water.
- Place both the plates in the copper sulphate solution.
- Connect the copper plate to the positive terminal of the battery and brass to the negative terminal.
- When we pass the current through the copper sulphate solution, it dissociates into copper and sulphate.
- The copper ions are deposited to the brass connected to the negative terminal of the battery.
- The loss of copper in the solution is restored by the copper plate, which gives an equal amount of copper to the solution, and the process continues.
- This means copper gets transferred to the brass plate.
- This is how copper is deposited on the brass plate through the solution.
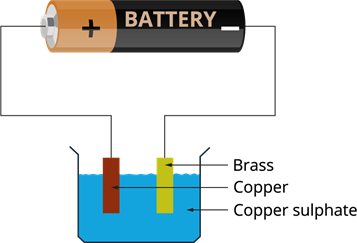
Electroplating of brass by copper
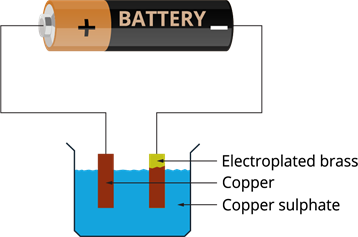
After electroplating: Brass is coated with copper
Application:
- Used for improving conductivity.
- Electrical resistance.
- Gold or silver coating can be done on the less expensive metals.
- Corrosion resister.
- Reduces friction.
- Before painting, it makes surfaces for better adhesion.
