PDF chapter test TRY NOW
The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
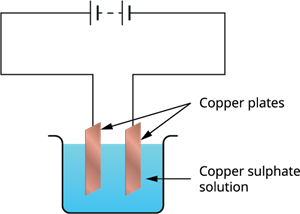
The thick rod of the plate is to be attached to the battery's positive terminal because the solution is dissociated into copper and sulphate when an electric current is passed through it. Since the free copper is charged, it is pulled to the negative terminal of the battery and deposits on it. The impure copper rod attached to the battery's, on the other hand, replaces the copper lost in the solution.
