
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAtoms are the fundamental particles of every object and material.

Objects around us
In our daily life, the materials we see, such as pen, paper, water can, stone, bus, car, even our nails, and hairs, are composed of more than a million atoms.
Though the atoms are very tiny and insignificant in size, should we ignore them?

Miniature people
Of course not, because our entire universe is made of these atoms. Though we could not see those atoms, it affects each and every action that we do.
We know that atoms are too small, but what is the size of an atom? Now, let us compare the size of atoms with some materials.
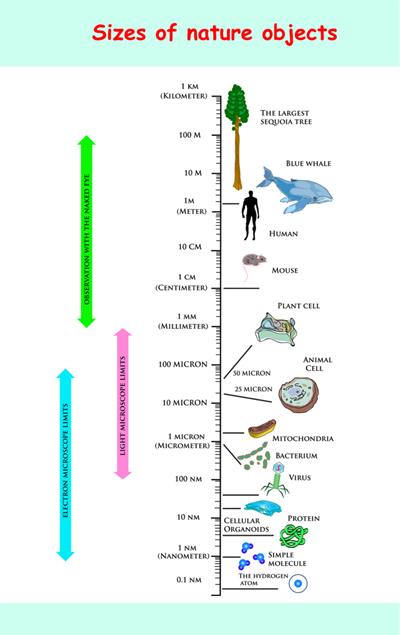
Size chart
S.No | Radii (in meter) | Example |
1 | Hydrogen atom | |
2 | Water molecule | |
3 | Molecule of haemoglobin | |
4 | Grain of sand | |
5 | Size of an ant | |
6 | Watermelon |
Atoms:
Atoms are incredibly tiny, smaller than anything we can imagine or compare them to. When millions of atoms are stacked together, they form a layer just as dense as a thin sheet of paper.

We learned that everything in our surroundings is made up of atoms. There would be no air, food or clothing, etc. without these atoms; indeed human body is also made up of atoms.
Atom size
Biometric Identification
We all have a name, and we named the materials and objects in our surroundings to identify them. Similarly, scientists also named atoms and have given unique symbols to identify them.
Now let's take a look at those symbols of the elements.
Symbols of elements:
Jöns Jacob Berzelius, a Swedish chemist, proposed that element symbols be made up of one or two letters from the element's name.

Jöns Jacob Berzelius
We can see that each element has a name and a unique chemical symbol. Some elements' symbols are made up of the first letter of the name and a letter that appears later in the name.
But John Dalton was the first scientist to use the symbols for elements in a very specific sense. Below we see the symbols of the atoms proposed by John Dalton.
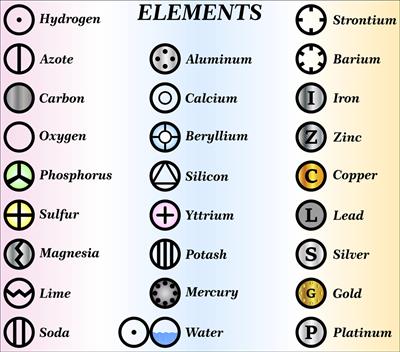
We can see that each element has a name and a unique chemical symbol. Some elements' symbols are made up of the first letter of the name and a letter that appears later in the name.
Example:
i). Hydrogen - H
ii). Magnesium - Mg
But some elements named after another language like Greek and Latin, so those elements would be different from others.
Example:
i). The symbol of iron is Fe from its Latin name Ferrum (Fe).
ii). Potassium symbol is K from Kalium (K).
iii). Sodium is Na from Natrium (Na).
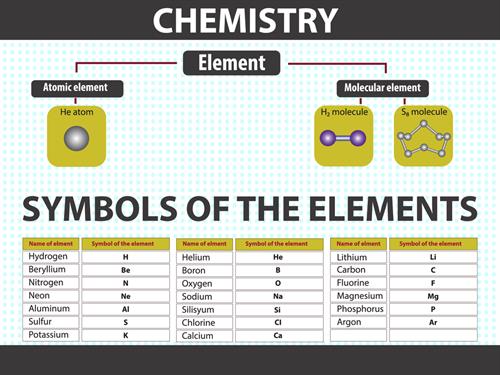
Nowadays, the names of elements are authorised by the IUPAC (International Union of Pure and Applied Chemistry).