PDF chapter test TRY NOW
Have you ever bought a mixture from a bakery? If you noticed closely, you would have seen a variety of edible items inside that cover.

Bakery mixture
Mixture:
Similar to the above example, a mixture is nothing but a combination of two or more substances. A mixture is a substance made of two or more kinds of elements or compounds or both, physically mixed in.
Example:
Sand water
Oil in water
Chalk in water
Sugar solution
Saltwater
Fruit juice
Oil in water
Chalk in water
Sugar solution
Saltwater
Fruit juice
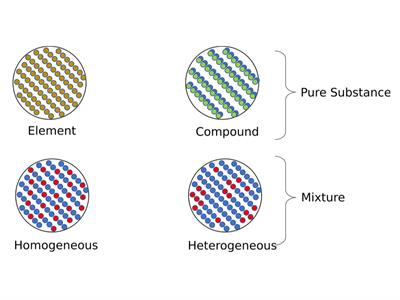
Structure of Pure and Mixture Substance's
Homogeneous Mixture:
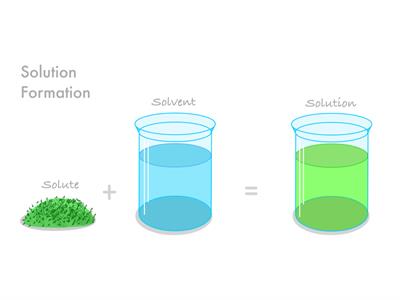

- All of the mixture's components are blended evenly.
- There are no visible separate barriers.
- There is just a single-phase solution.
- Salt in water is the best example.
Heterogeneous Mixture:
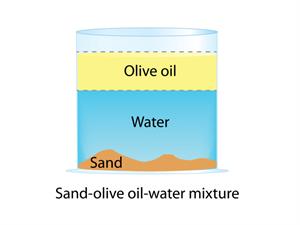

- All of the mixture's components aren't evenly distributed.
- There are noticeable separate lines.
- There is two or more phase in the solution.
- Air, sand, and table salt are examples.
In a heterogeneous mixture, components are visible to the naked eye. And it does not have a uniform composition and properties.
Example:
Sand water
Oil in water
Chalk in water
Oil in water
Chalk in water
Experiment to prove heterogeneous mixture:
- Step 1: Take some water and oil in a vessel. Stir it well.
- Step 2: Get some sand and mix it in the water.
Result: You can see that the sand in the water and the oil in the water are not so mixed well. So, these kinds of mixture with uneven compounds known as heterogeneous mixture.
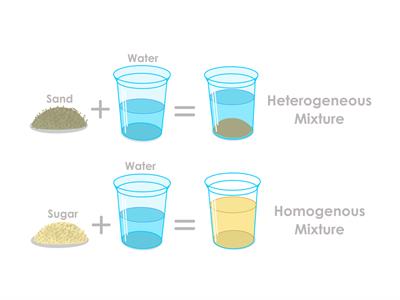
Experiment to prove homogeneous mixture:
- Step 1: Take some water in a bowl.
- Step 2: Add any compound in that water, such as sugar or salt.
- Step 3: Mix the water with sugar or salt uniformly.
Result: Now, the obtained solution is the homogenous mixture. Homogenous mixtures are made by mixing any two or more compounds in the same proportions.
