PDF chapter test TRY NOW
Do you know why lemonade tastes the same throughout the drink?
Why and how does this happen?
Whenever we drink juice, it tastes the same throughout. This is because, it shows that particles of sugar or salt evenly distributed in the solution (juice).

Lemon Juice
Solutions:
A solution is a mixture of two or more substances that appears to be uniform in appearance. A solution is a homogeneous mixture of two or more substances.

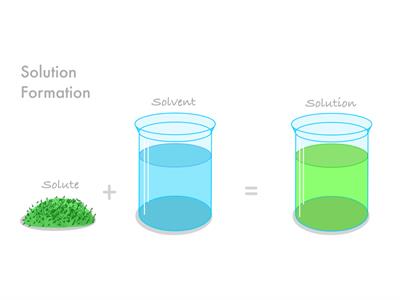
A solution is a mixture in which one substance dissolves fully in the other. The solute is the material that dissolves. The solvent is the substance that does not dissolve.
A solute is a part of a solution that is present in a smaller quantity by weight.
A solvent is a variable that is present in a greater quantity by weight.
If we add the solvent and solute, we get the solution.
Solution = Solvent + Solute.
Types of Solutions:
Various types of solutions are-
- Solids in a solid solution: Alloys
- Solids in a liquid solution: Salt solution, sugar solution.
- Liquids in a liquid solution: Lemon extract in water.
- Gas in a gas solution: Natural gas, air.
- Gas in a liquid solution: Carbonated drinks.
Example:
1. Solids in a liquid solution: Salt solution, sugar solution.

Solute: Salt is the solute.
Solvent: Water is the solvent.
2. Gas in a liquid solution: Aerated drinks.

Solute: Carbon dioxide (gas) is the solute.
Solvent: Water (liquid) is the solvent.
3. Solids in a solid solution: Alloys.
Alloys are mixtures of two or more metals, a metal and a non-metal, that can't be segregated into their constituent parts using physical methods.

Objects made from Brass
However, since an alloy exhibits its constituents' properties and may have a variable composition, it is still a mixture. Brass, for example, is made up of about 30\% zinc and 70\% copper.
4. Liquids in a liquid solution: Lemonade.

Solute: Lemon extract is the solute.
Solvent: Water is the solvent.
Solvent: Water is the solvent.
5. Gas in a gas solution: Natural gas, air.
Air is an example of a mixture in a gaseous solution. Air is a gaseous mixture that is homogeneous in composition.

Wind
Oxygen 21\% and nitrogen 78\% are the two primary constituents present in air. The other gases are only found in trace amounts. Natural gas is also an example of this type of gas in a gas solution.
