
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn the evaporation process, when the heat is applied, the water changes into water vapour.
When the water is boiled for some time, its quantity will decrease by changing into water vapour.
The evaporation used to separate the substance from the solvent or to obtain concentrated solutions.
Example:
1. Water boiling in a kettle.
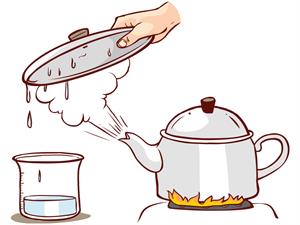
Boiling water
2. Water cycle.

Water cycle
Evaporation is the transitional phase of an substance as it changes liquid phase to the gas phase that takes place below the boiling temperature. So, evaporation starts at below the boiling temperature of the substance.
Now we get an idea about the evaporation. Let's move on to what are the things that can affect the evaporation.
Evaporation is a phenomenon acting on the surface. The evaporation process increases with the following:
1. An increase of a surface area.
If the surface area of the liquid layer is increased, the rate of evaporation increases.
For example, while putting clothes for drying up, we spread them out.
2. An increase of temperature.
When the temperature of the liquid increased, the particles in the liquid gets enough kinetic energy to reach the liquid to vapour state.
3. A decrease in humidity. (the amount of water vapour present in air)
When the amount of water in the air significantly high, it decreases the evaporation.
For example, in the cold region, the evaporation process would be slow due to high humidity.
4. An increase in wind speed.
We might have seen that when the wind speed is high, the wet clothes will dry faster. As the wind speed increases, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.

Evaporation