PDF chapter test TRY NOW
In nature, a number of atoms of certain elements have been discovered with the same atomic number but different mass numbers. Here are some examples:
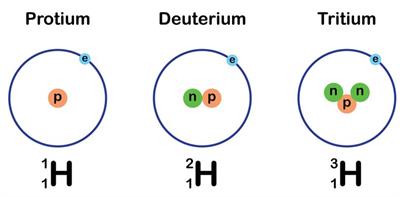
A hydrogen atom with same atomic number but a different mass number
|
|
Name
|
Atomic No.
|
Mass No.
|
|
or H
|
Protium
|
\(1\)
|
\(1\)
|
|
or D
|
Deuterium
|
\(1\)
|
\(2\)
|
|
or T
|
Tritium
|
\(1\)
|
\(3\)
|
Other examples:
|
|
Name
|
Atomic No.
|
Mass No.
|
|
, ,
|
Carbon
|
\(6\)
|
\(12\), \(13\), \(14\), respectively
|
|
,
|
Chlorine
|
\(17\)
|
\(35\), \(37\), respectively
|
Isotopes:
We observed that some elements have the same atomic numbers but different mass numbers. These are known as isotopes.
Most of the element are consist of a mixture of isotopes. They are pure substances. Their chemical properties are similar, but their physical properties are not.
In nature, chlorine exists in two isotopic forms, with masses of \(35\ u\) and \(37\ u\) in a \(3:1\) ratio. Obviously, the question that arises here is what mass of chlorine atom we can use. Let's see what happens.
The atomic mass of a given element is a weighted average of its isotopes. Each isotope's mass is divided by its abundance.
Based on the above information, the average atomic mass of a chlorine atom will be,
\(=\)
\(=\) 35.5
The total mass of all naturally occurring atoms of any natural element is taken as the mass of an atom of that element. If an element does not have any isotopes, the mass of the atom is equal to the sum of its protons and neutrons. However, if an element exists in isotopic forms, we must first calculate the percentage of each isotopic form before calculating the average mass.
This does not indicate that each chlorine atom has a \(35.5\ u\) fractional mass. It indicates that a given amount of chlorine would contain both isotopes of chlorine with an average mass of \(35.5\ u\).
Application of Isotopes:
- The age of fuels and other organisms is determined using carbon isotopes.
- The ratio of \(C\)-\(14\) to \(C\)-\(12\) is established. When an organism dies, the \(C\)-\(14\) starts to decompose, while the \(C\)-\(12\) does not. As a result, as the age of the dead organism gets older, the percentage of C-\(14\) decreases. The age of the organism can be calculated using this percentage.
- Co-\(60\) is a radioactive isotope. It decays by emitting gamma rays, which are used to destroy cancer cells.
- In the industry, gamma radiation can find that canned foods and bottled drinks are either fully filled or partially.
- In the treatment of goitre, an isotope of iodine is used.
- Blood flow is traced with Na-\(24\) to identify whether there is an obstruction.
