PDF chapter test TRY NOW
J. J. Thomson introduced an atom model, which helped in laying the foundation for further research in the atomic model. Significantly the research on the atomic model was done by Rutherford.
Rutherford Model:
Ernest Rutherford was curious about the arrangement of electrons in an atom. He designed an experiment in which alpha particles were used to fall on a thin gold foil. This experiment is also known as the Alpha particle scattering experiment.
Source:
Rutherford used alpha particles (also called alpha rays or alpha radiation) as the source.
Alpha particle:
- Alpha particles are helium ions with two charges
- Fast-moving particles
- High energy
- Heavier than protons
- Symbol is or or
The radioactive source of alpha particles were kept in a lead box with a small hole. Hitting particles were stocked by the box and passed through the hole inside the box.
Why Rutherford used alpha particles for this experiment?

As Thomson proposed, if the atom is a pudding of positive charge with electrons embedded in it, the particles pass straight through it because heavier particles will pass through a lighter pudding structure of the atom.
Gold foil:
He chose gold foil because he wanted the layer to be as thin as possible. The thickness of this gold foil was about \(1000\ \)atoms.
Detector:
The detector is a device that allows scientists to see what is happening in an experiment.
Rutherford used a circular fluorescent screen (coated with zinc sulphide, \(ZnS\)) as the detector.
When particles strike it, this detector glows or emits fluorescent light.
Experiment:
- The alpha particles that pass through the box's hole are constrained to a straight line.
- They hit the gold foil.
- The scattered alpha particles were detected by the detector.
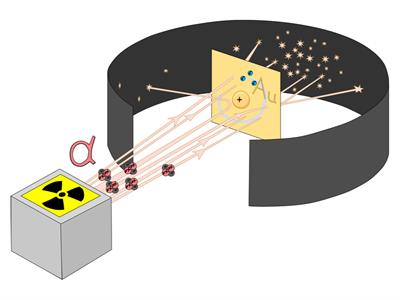
Figure \(1\): Scattering of \(α\)-particles by a gold foil
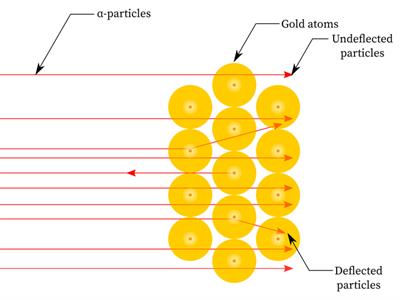
Figure \(2\): Scattering of \(α\)-particles by a gold foil
Observation:
- Since most alpha particles passed through the gold foil without being deflected, most of the space inside the atom is empty.
- Only a few particles were deflected from their direction, suggesting that the atom's positive charge uses very little space.
- Just a small percentage of alpha particles were deflected by \(180°\), showing that the gold atom's positive charge and mass were concentrated in a very small volume within it.
He proposed the nuclear model of the atom based on these observations. This experiment results in a more detailed definition of an atom.
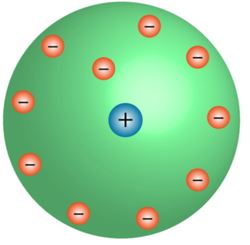
Rutherford's model of an atom
Features of the atom:
- The positive centre of the atom is known as the nucleus.
- All the mass is concentrated on the nucleus, around which the electrons circulate in the well-defined orbit, much like planets revolving around the Sun.
- The size of the nucleus is less than the atom.

Rutherford
He was known as the ‘Father’ of nuclear physics.
For this theory, he was awarded the Nobel prize for chemistry in the year \(1908\).
For this theory, he was awarded the Nobel prize for chemistry in the year \(1908\).
Drawbacks of Rutherford's atomic model:
As electrons revolve in orbit, they accelerate and lose energy. After that, they fall into the nucleus. If this happened, the atom would no longer be stable, and the matter would no longer exist in the way we know it. Atoms are known to be very stable. Thus, Rutherford's model failed to explain the stability of the atom.
