PDF chapter test TRY NOW
The structural frameworks of organic compounds are composed of carbon and hydrogen, which are less reactive. However, the presence of another atom or group of atoms makes the compounds more reactive, determining the chemical properties of the compounds. These are known as functional groups.
The chemical properties of organic compounds are determined by its functional group, whereas the remaining structure of the compound determines its physical properties.
Carbon's multiple bonds (C=C, C\equiv C) are also considered functional groups as they influence many properties. Other functional groups are –OH, –CHO, –COOH, and so on.
A functional group is an atom or group of atoms in a molecule that determines its chemical properties.
Example:
1. Alcohol:
Ethane is a hydrocarbon with the molecular formula C_2H_6. If one of its hydrogens is replaced by a –OH group, an alcohol is formed. After leaving the functional group, 'R' represents the rest of the structure. As a result, 'R-OH' represents alcohol.

Ethane and ethanol
Name | Molecular formula |
| Methanol | CH_3OH |
| Ethanol | CH_3CH_2OH |
| Propanol | CH_3(CH_2)_2OH |
| Butanol | CH_3(CH_2)_3OH |
| Pentanol | CH_3(CH_2)_4OH |
List of alcohols with the increase in 'R' group
Example:
2. Aldehyde:
Pentane is a hydrocarbon with the molecular formula C_5H_12. If one of its hydrogens is replaced by a –CHO group, an aldehyde is formed. After leaving the functional group, 'R' represents the rest of the structure. As a result, 'R-CHO' represents aldehyde.
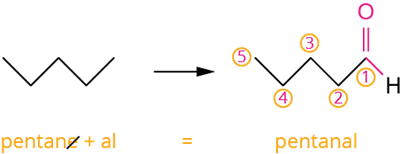
Pentanal
Name | Molecular formula |
| Methanal | HCHO |
| Ethanal | CH_3CHO |
| Propanal | CH_3CH_2CHO |
| Butanal | CH_3(CH_2)_2CHO |
| Pentanal | CH_3(CH_2)_3CHO |
List of aldehydes with the increase in 'R' group
A class of organic compounds is a group of compounds that share the same functional group.
The below table depicts various classes or families of organic compounds as well as their functional groups:
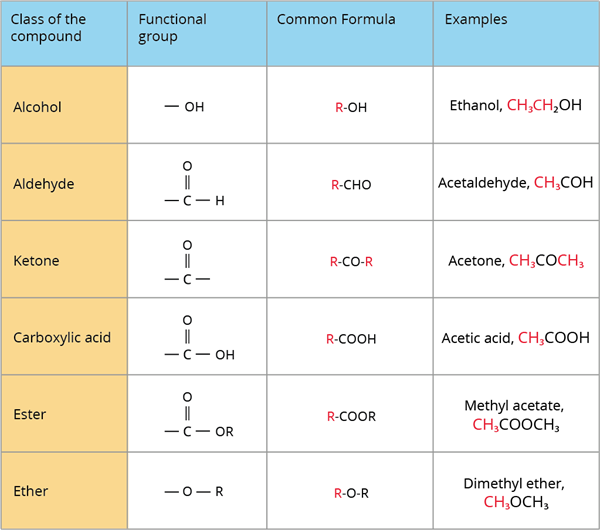
Classes of organic compounds based on functional group
