PDF chapter test TRY NOW
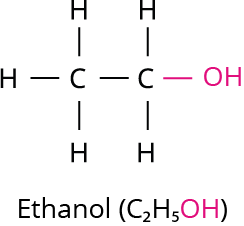
Ethanol is generally referred to as alcohol. All alcoholic liquids and some cough syrups contain ethanol. The molecular formula for ethanol is C_2H_5OH.
Skeletal structure of ethanol
Ethanol is prepared in industries via the fermentation of molasses, obtained through sugar from sugarcane. Molasses is a dark coloured syrupy liquid left after the crystallisation of sugar from the concentrated sugarcane juice. Molasses contain approximately 30% of sucrose, which cannot be separated by using crystallisation.
Sucrose is therefore converted into ethanol by using the subsequent steps:
- Dilution of molasses
- Addition of nitrogen source
- Addition of yeast
- Distillation of 'Wash'
a) Dilution of molasses:
Molasses is first diluted with water to decrease the concentration of sugar to approximately 8-10\%.
b) Addition of nitrogen source:
During the fermentation process, the nitrogenous matter that is present in molasses acts as food for yeast. Ammonium sulphate or ammonium phosphate is added if the nitrogen content of the molasses is poor.
c) Addition of yeast:
The solution extracted in addition of nitrogen source is collected in large ‘fermentation tanks’, and yeast is mixed. The aggregate is preserved at 303K for some days. All through this period, the enzymes invertase and zymase found in yeast result in the conversion of sucrose into ethanol.
(i) \text{Sugar + Water \xrightarrow {invertase} Glucose + Fructose}
C_{12}H_{22}O_{11} + H_2O \xrightarrow{invertase} C_6H_{12}O_6 + C_6H_{12}O_6
(ii) \text{Glucose or Fructose \xrightarrow {zymase} Ethanol + Carbon dioxide}
C_6H_{12}O_6 \xrightarrow{zymase} 2C_2H_5OH + 2CO_2
The fermented liquid from this process is called as 'Wash'.
d) Distillation of wash:
The fermented liquid (i.e. wash), containing 15% to 18% alcohol, is now subjected to fractional distillation. The primary fraction drawn is an aqueous solution of ethanol. 95.5% of ethanol and 4.5% of water proportion is known as rectified spirit.
Rectified spirit is then refluxed over quicklime for about 5 to 6 hours and then allowed settling for 12 hours. Pure alcohol (100%) is obtained on the distillation of the above mixture. This is known as absolute alcohol.
In the next section, we will see the properties and uses of ethanol (C_2H_5OH).
