
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoEthanoic acid (CH_3COOH), commonly known as acetic acid, is one of the most important compounds of the carboxylic acid circle of relatives. Its molecular formula is C_2H_4O_2. The structural formula for ethanoic acid is,
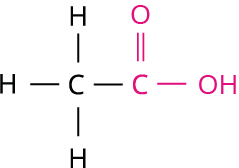
Ethanoic acid
Preparation of ethanoic acid:
Ethanoic acid is prepared on a large scale through the oxidation of ethanol in the presence of alkaline potassium permanganate or acidified potassium dichromate.
Physical properties of ethanoic acid:
- Colourless liquid with a strong odour.
- Acids are sour in taste, and so is ethanoic acid.
- They are miscible in water irrespective of their proportions.
- They have a higher boiling point than the corresponding groups of aldehyde, alcohol and ketones.
- Pure ethanoic acid on cooling forms ice-like flakes. As it resembles glaciers it is known as glacial acetic acid.
Chemical properties of ethanoic acid:
a) Reaction with metal:
The reaction of active metals (such as Na, Zn, etc.) with ethanoic acid forms ethanoate with the liberation of hydrogen gas.
2CH_3COOH + Zn \rightarrow (CH_3COO)_2Zn + H_2
Zinc ethanoate
2CH_3COOH + 2Na \rightarrow 2CH_3COONa + H_2
Sodium ethanoate
b) Reaction with carbonates and bicarbonates:
Ethanoic acid reacts with weaker bases such as sodium carbonate (Na_2CO_3) and sodium bicarbonate (NaHCO_3), forming its salt, water along with the liberation of brisk effervescence (CO_2).
2CH_3COOH + NaCO_3 \rightarrow 2CH_3COONa + CO_2 + H_2O
Sodium ethanoate
CH_3COOH + NaHCO_3 \rightarrow CH_3COONa + CO_2 + H_2O
Sodium ethanoate
c) Reaction with base:
Sodium ethanoate and water is formed when ethanoic acid reacts with sodium hydroxide.
CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O
Sodium ethanoate
d) Decarboxylation (removal of CO_2):
The reaction of soda lime (solid mixture of 3 parts of NaOH and one part of CaO) with the sodium salt of ethanoic acid produces methane gas.
CH_3COOH \xrightarrow {NaOH /CaO} CH_4+ Na_2CO_3
Methane
gas
Uses of ethanoic acid:
- Lower concentrations of acetic acid (vinegar) are used as a food additive, flavouring agent, and preservative.
- They are used in the manufacturing of plastics.
- Used in making dyes, pigments and paints.
- It is used as a laboratory reagent and for printing on fabrics.
- They are also used for coagulating rubber from latex.
- Ethanoic acid is used in the production of pharmaceuticals.