
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAlpha decay:
Alpha decay is a nuclear reaction in which an unstable parent nucleus emits an alpha particle and forms a stable daughter nucleus.
The decay of Uranium (U^{238}) to Thorium (Th^{234}) with the emission of an alpha particle.
As the parent nucleus emits an α -\ particle during the decay, it is evident that the mass number of the daughter nucleus reduces by four and the atomic number decreases by two.
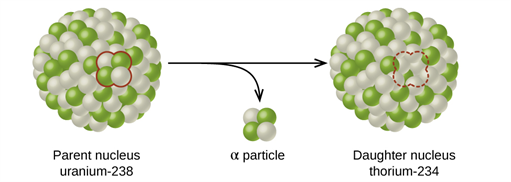
Alpha decay process
Beta decay:
Beta decay is a nuclear reaction in which an unstable parent nucleus emits a beta particle and forms a stable daughter nucleus.
Beta decay of phosphorous is a good example.
In β-\ decay, the atomic number increases by one, but there is no change in the mass number of the daughter nucleus.
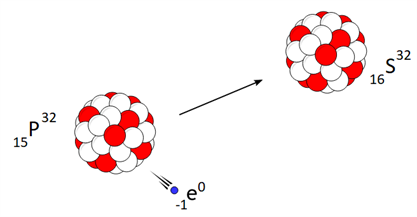
Beta decay process
Important!
NOTE: The atomic number of the resulting nucleus identifies the product nucleus in a nuclear reaction.
Gamma decay:
In gamma decay, only the energy level of the radioactive nucleus changes, but the atomic number and mass number remains the same.
Gamma decay process
In radioactive decay, both α-decay and β-decay never take place together in a single process. Either α-decay or β-decay occurs along with the emission of γ particles.
Reference:
https://upload.wikimedia.org/wikipedia/commons/3/37/Gamma_Decay01.svg