
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAluminium, copper and iron are the most abundant metals found in the Earth's crust. These metals are most widely used in various industries due to their versatility, tensility, and hardness.
Although aluminium compounds have been used for thousands of years, we had extracted aluminium metal for the first time around 170 years ago.
Although aluminium compounds have been used for thousands of years, we had extracted aluminium metal for the first time around 170 years ago.

Aluminium, copper and iron utensils
Furthermore, these metals exhibit the following physical properties. The properties are as follows;
- Metals like copper and iron are hard, while aluminium is soft.
- Even more, these metals are malleable, ductile, corrosion-resistant and have high electrical conductivity.
These metals are widely used for various industries such as automobile, aviation, railways, construction, electrical and electronics.
Aluminium and copper are the lightest metals, with a strength-to-weight ratio higher than that of steel.
The favourable properties such as strength, lightness, corrosion resistance, recycling, and moulding make aluminium and copper the most widely used metals.
Aluminium and copper are the lightest metals, with a strength-to-weight ratio higher than that of steel.
The favourable properties such as strength, lightness, corrosion resistance, recycling, and moulding make aluminium and copper the most widely used metals.
This topic will help us update our knowledge of how aluminium, copper, and iron metals are separated and used.
Some basic questions for your knowledge and understanding.
No. | Questions | Answers |
1 | I am the most abundant metal in the Earth's crust; I also appearing silvery-white colour. Guess who I am? | Aluminium |
2 | I am a necessary part of your home light. You cannot make lightning without my help. Do you know who I am? | Electric cables (copper) are used for the power supply. |
3 | I am a significant part of Hemoglobin; I also use to holding a ship. Can you guess who I am? | Iron is a significant part of Hemoglobin and is used for making anchors. |
Let's now see the development of Aluminium, Copper and Iron and their properties, uses.
Metallurgy of Aluminium:

Aluminium symbol, atomic number and mass.
The most abundant metal in the Earth's crust is aluminium. It exists in the combined state because it is a reactive metal.
India's largest bauxite producing state is Odisa (51%), followed by Andhra Pradesh (16%), Gujarat (9%), Jharkhand (6%), Maharashtra (5%). Primary bauxite resources are concentrated in the East Coast Regions.
The primary ores of aluminium are as follows:
Ores of Aluminium | Formula |
| Bauxite | Al_2O_3.2H_2O |
| Cryolite | Na_3AlF_6 |
| Corundum | Al_2O_3 |
The chief ore of aluminium is bauxite.
Bauxite is a reddish clay substance that has been transformed into a rock. It contains aluminium oxide (alumina), silica, iron oxides and titanium dioxide compounds.
Aluminium is extracted from bauxite in two steps:
Step (i). Bauxite to alumina conversion - Bayer’s Process:
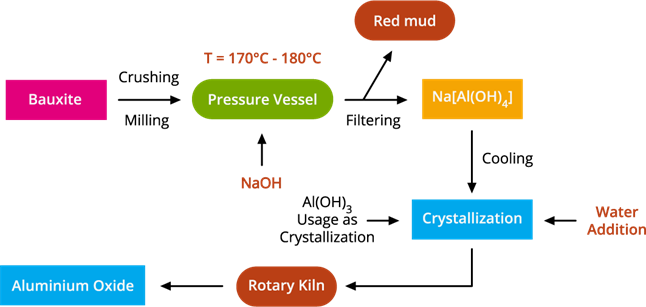
The flowchart of Bayer's process
The Bayer process is the leading industrial process of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer.
The first step in this process is to crush the bauxite ore into a fine powder heated under pressure with a concentrated sodium hydroxide solution at (150)°C to produce sodium meta-aluminate as red mud, which is then crystallized with water to form aluminium hydroxide crystals. Then the final step of the Bayer process is to ignite the crystals in a rotatory kiln evaporator at (1000)°C, followed by filtering, washing, and drying the residues to get alumina.
The first step in this process is to crush the bauxite ore into a fine powder heated under pressure with a concentrated sodium hydroxide solution at (150)°C to produce sodium meta-aluminate as red mud, which is then crystallized with water to form aluminium hydroxide crystals. Then the final step of the Bayer process is to ignite the crystals in a rotatory kiln evaporator at (1000)°C, followed by filtering, washing, and drying the residues to get alumina.
Bauxite to alumina conversion involves the following stages:
Stage 1: Bauxite ore is finely powdered and heated under pressure with a concentrated caustic soda (Sodium hydroxide) solution at 150°C to produce sodium meta-aluminate.
Al_2O_3 + 2NaOH → 2NaAlO_2 + H_2O
Bauxite + Caustic soda → Sodium meta-aluminate.
Stage 2: When sodium meta-aluminate is diluted with water, aluminium hydroxide precipitate is formed.
NaAlO_2 + 2H_2O → Al(OH)_3 + NaOH
Sodium meta-aluminate + Water → Aluminium hydroxide.
Stage 3: Alumina is produced by filtering, washing, drying, and igniting the precipitate at 1000°C.