
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoElectronegativity:
An element's electronegativity measures its atom's tendency to attract the shared pair of electrons towards itself in a covalent bond.
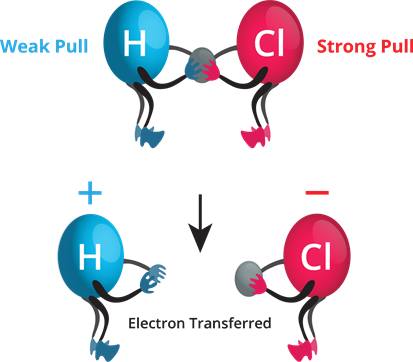
- Consider the HCl molecule. The hydrogen and chlorine atoms give one electron each to form a covalent bond.
- A chlorine atom has a higher electronegativity, and hence it pulls the shared electrons towards itself more strongly than hydrogen. Thus, the bonding electrons are left with chlorine, forming H^+ and Cl^– ions when the bond breaks.
Relative electronegativity of H and Cl.
- Electronegativity is determined by various experimental data, including bond energy, ionisation potential, electron affinity, etc.
- The Pauling scale is the most used scale for determining electronegativity, which predicts the type of bonding (ionic or covalent) between atoms in a molecule.
Some of the elements' electronegativity is listed below.
F = 4.0, Cl = 3.0, Br = 2.8, I = 2.5, H = 2.1, Na = 1
- If the electronegativity difference between two elements is 1.7, then the bond has 50% ionic character and 50% covalent character.
- The bond is considered more covalent if the difference is less than 1.7.
- If the difference is more than 1.7, the bond is considered more ionic.
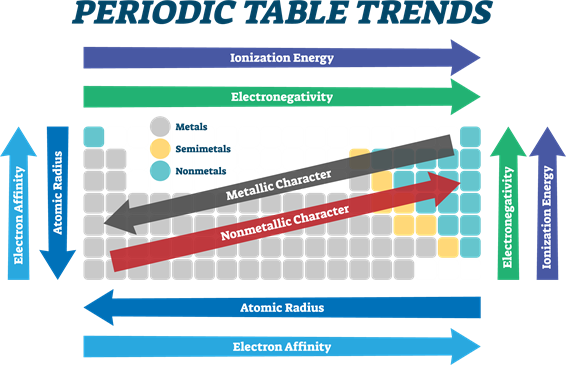
- From left to right in the periodic table, the electronegativity increases due to an increase in nuclear charge, which in turn attracts electrons more strongly.
- Conversely, on moving down a group, the electronegativity of the elements decreases because of the increased number of valence shells.
Periodic Property | In Periods | In Groups |
| Atomic radius | Decreases | Increases |
| Ionic radius | Decreases | Increases |
| Ionisation energy | Increases | Decreases |
| Electron affinity | Increases | Decreases |
| Electronegativity | Increases | Decreases |