
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoElements Introduction:
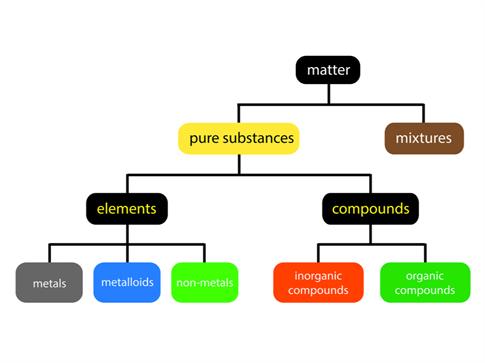
Everything in this universe is made of matter. We live in a world of substances with great diversity. The combination of various elements produces substances. All the elements are unique in their nature and property. Over the years, scientist have looked for different ways to categorize these elements according to their properties.
(a). Do you know who I am? I am the highest table in chemistry.
(b). Without me, there is no chemical substance.
(c). Why I only arranged in table format?
(d). Do you know how many elements have been discovered so far?
Did you get all the answers to the above asked questions? if not, here are the correct answers.
(A). Periodic table, (B). Elements, (C). Easy identification, (D). 118.
Let's us now see the development and the classification periodic table.
Let's us now see the development and the classification periodic table.
The 18th and 19th centuries observed fast development in chemistry in all scientific activities. By 1860, scientists had already found 60 elements and calculated their atomic masses.
They saw that some elements had comparable properties and hence arranged them into groups. During this period, scientists discovered many new elements with wide range of properties.
Scientists realised that it would be more convenient to read the properties of these elements in groups and periods rather than individually.

In this way, each group that contained a certain number of elements with similar properties (such as an arrangement of fruits and vegetables demonstrating orderliness) and periods expressed a regular gradation. So, scientists made many attempts to organize this elements logically.

In continuation of the knowledge gained in the topic periodic classification of elements with earlier concepts and their subsequent deliberations, now you are ready to move on to higher-order thinking to improve your understanding of properties of element.