PDF chapter test TRY NOW
We know what solubility is. Let us now see the factors affecting the solubility:
Solubility refers to the maximum amount of a substance that can be dissolved in a given volume of solvent. Solubility in water is frequently expressed in gram/100mL.
The solute's solubility is governed by three major factors:
- Nature of the solute and solvent
- Temperature
- Pressure
1. Nature of the solute and solvent:
Solubility is greatly influenced by the nature of the solute and solvent. Although water dissolves a wide range of ionic and covalent substances, it does not dissolve everything.
When predicting solubility, scientists frequently use the phrase "like dissolves like." This expression means that dissolving occurs when the solvent and the solute have similarities.
For instance, common salt is a polar compound that dissolves easily in a polar solvent such as water.
In non-polar solvents, non-polar compounds are soluble. For example, fat dissolved in ether.
Non-polar compounds, on the other hand, do not dissolve in polar solvents, and polar compounds do not dissolve in non-polar solvents.
2. Temperature:
(a) Solubility of Solids in Liquid:
In general, the solubility of a solid solute in a liquid solvent increases as the temperature rises.
Example:
Sugar dissolves more readily in warm water than in cold water.
Solubility increases with increasing temperature in an endothermic process.
Solubility decreases with increasing temperature in an exothermic process.
(b) Solubility of Gases in Liquid:
Do you know why water bubbles form when water is boiled?
With increasing temperature, the solubility of gases in liquids decreases causing it to evaporate forming bubbles.
In general, water contains dissolved oxygen. When water is heated, the solubility of oxygen in water decreases, allowing oxygen to escape in the form of bubbles.
Aquatic animals thrive in colder climates because the water contains a higher concentration of dissolved oxygen. This demonstrates that oxygen is more soluble in water at low temperatures.
3. Pressure:
Only when gas is soluble in a liquid, the effects of pressure is observed. The solubility of a gas in liquid increases as pressure is increased.
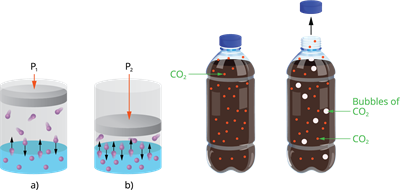
Effect of pressure on solubility
Example:
Carbonated beverages, i.e. soft drinks, household cleaners containing an aqueous solution of ammonia, formalin -aqueous solution of formaldehyde, and so on, are common examples of gas solubility in liquids.
Note: Henry's law describes the effect of pressure on the solubility of a gas in a liquid. It states that the solubility of a gas in liquid at a given temperature is directly proportional to the pressure of the gas over the solution.
