PDF chapter test TRY NOW
In the previous session, we have discussed the effect of heat energy.
In this session, we will discuss the changes of state in a substance due to heat.
When we apply the heat or thermal energy to the substance, the molecule's kinetic energy increases due to the supplied energy, increasing its temperature.
Example:
Water boiling and evaporating into water vapour (steam) when heated on the stove.
The applied energy is used only to separate the molecules; no part of it is used to rise the molecule's kinetic energy. There is no change in temperature unless a phase change is complete. i.e. during phase change.
Changes of Phase:
The term change of phase means the same thing as the term change of state. The change of phase always occurs with a change of heat. If heat energy is supplied to or taken out
from a substance, it will undergo a change from one state of matter to another state.However, the temperature does not change.
During a change in state, the heat energy is used to change the bonding between the molecules. There are changes in bonding energy between the molecules.
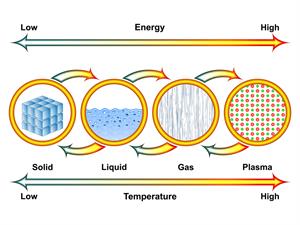
Change of state in matters
There are four states of matter in the universe - plasma, gas, liquid and solid. But, matter on Earth exists mostly in three distinct phases - gas, liquid and solid.
A phase is a distinctive form of a substance, and matter can change among phases.
One of the following transformations are possible,
- Solid to Liquid
- Liquid to Gas
- Solid to Gas
- Gas to Liquid
- Liquid to Solid
- Gas to Solid
Description of Phase change | Term for Phase change | Heat movement during Phase change | Temperature change during Phase change |
Solid to Liquid | Melting | Heat goes into the solid as it melts. | None |
Liquid to Gas | Vaporization, which includes boiling and evaporation | Heat goes into the liquid as it vaporizes. | None |
Solid to Gas | Sublimation | Heat goes into the solid as it sublimates. | None |
Note: Water is the only matter on the earth that can be found naturally in all three states - Solid, Liquid and Gas.
