
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn this session, we are going to discuss the concept of thermal equilibrium. To get a clear idea about thermal equilibrium, you need to know the basics of heat flow.
Heat Flow:
Heat flow is the energy transfer process in which energy is transferred from one object to another object.
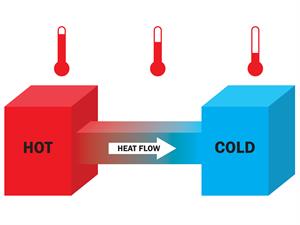
Heat flow
Whenever two systems come into thermal contact, one system's energy is transferred to another to reach thermal equilibrium. Here, heat or thermal energy is transferred between the objects. Generally, the temperature determines the direction of flow. In this case, the heat is transferred from the hotter object to the colder object.
Process of heat transfer from a hotter object to a colder object:
When two objects come into thermal contact, the heat energy of the hotter object is transferred to the colder object, which causes an increase in kinetic energy. Due to this rise in kinetic energy, molecules in the colder object start to move rapidly, resulting in an increase in temperature. This cycle repeats until thermal equilibrium is reached.
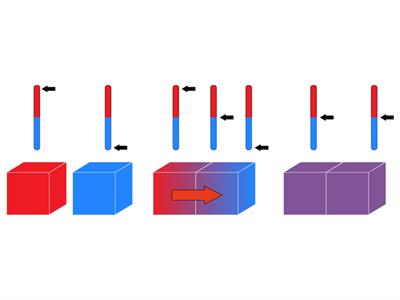
Heat transfer from a hot body to a cold body
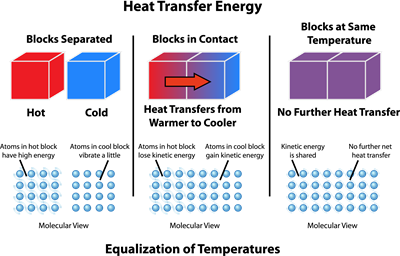
Heat energy transfer - description
The above diagram shows how the heat energy transfer takes place between two objects.
The diagram is divided into three columns.
- The first column represents that two objects with different temperatures and are not in thermal contact.
- The second column represents that the two objects are in thermal contact, and the heat transfers from a hot object to a cold object. It also represents that the atoms in the hot body lose their kinetic energy and transfer to the cold body.
- The third column represents that thermal equilibrium is achieved, and hereafter, there is no transfer of heat energy.
Thermal Contact:
Two systems are said to be in thermal contact if they can exchange energy through the process of heat transfer.
Thermal Equilibrium:
Two systems are said to be in thermal equilibrium if there is no transfer of heat between them, and both have the same temperature.