PDF chapter test TRY NOW
Osmosis:
The movement of solvent or water molecules from a region of higher concentration to a region of lower concentration region through a semi-permeable membrane is known as osmosis.
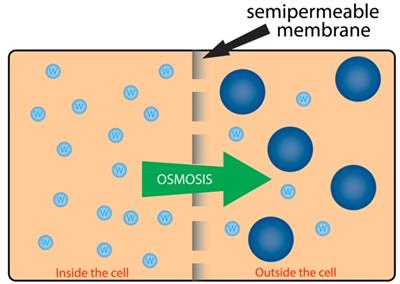
Osmosis
This process occurs till equilibrium is attained. It is a passive process (energy is not utilised) in which the movement of water or any other solvents is observed.
Types of solutions:
Based on the concentrations of solute, solutions are of three types:
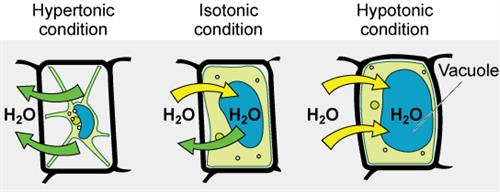
Types of solutions
1. Hypertonic: When the concentration of the external environment of the cell is greater than the environment inside the cell, it is known as hypertonic.
2. Isotonic: When the external and internal environments of the cell are equal, it is known as isotonic.
3. Hypotonic: When the concentration of the internal environment of the cell is higher than the external environment, it is known as hypertonic.
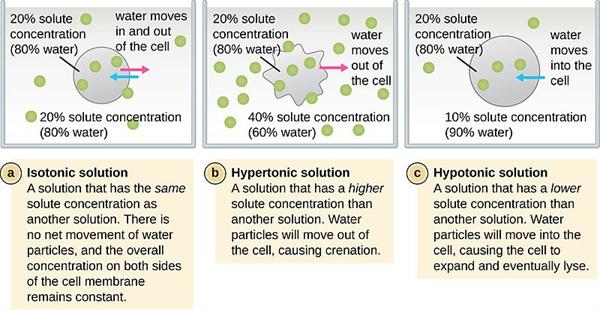
Various conditions of solutions
Plasmolysis:
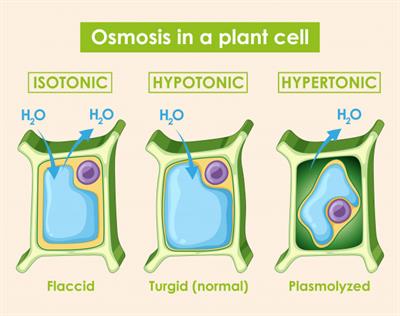
Plasmolysed cell
It is a process that occurs when a living plant cell is placed inside a hypertonic solution. During this, the water molecule moves out of the cell resulting in the shrinkage of protoplasm away from the cell wall.
Plasmolysis
Reference:
https://www.freepik.com/free-vector/diagram-showing-osmosis-plant-cell_5982972.htm
