PDF chapter test TRY NOW
Although pure water is commonly considered an electrical non-conductor, precise experiments revealed that it does carry electricity to some extent. This conductivity of water has resulted from the self-ionisation of water. A reaction in which two identical molecules react to produce ions is known as self-ionisation or auto ionisation.
During the ionisation of water, a proton from one water molecule is transferred to another water molecule, leaving an \(OH\)— ion behind. The proton dissolves in water and forms the hydronium ion, as shown in the equation below:
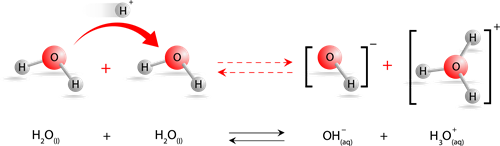
Hydronium ion formation
The hydroxyl ion is a strong base, whereas the hydronium ion is a strong acid. As a result, both hydroxyl and hydronium ions react quickly to produce water. Thus, it is a reversible reaction and reaches equilibrium very quickly. So, the extent of ionisation is minimal, and the concentration of the ions produced is also very less.
The product of the concentration of the hydronium ion and the hydroxyl ion is called ‘Ionic product of water’. It is denoted by '\(K_w\)'.
\(H_3O^+\) can be denoted as \(H^+\). Hence, the ionic product of water is
Its unit is \(mol^2\) \(dm^{-6}\). At \(25°C\), its value is \(1.00\times10^{-14}\).
