
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoChemistry is all about chemical reactions. As we have studied earlier, a chemical reaction involves the breaking of existing chemical bonds and the formation of new chemical bonds.
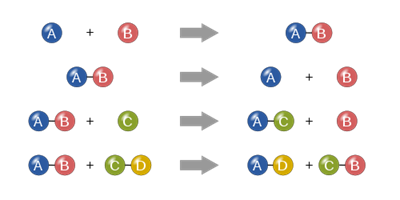
Chemical reactions
This change may occur naturally or be influenced by external forces or energy.
In our daily lives, we observe many chemical reactions. So, chemistry mainly focuses on chemical reactions.
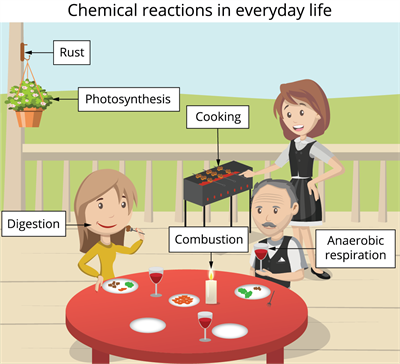
Chemical reactions in daily life
Let us see some examples:
We require energy to play, walk, run or perform other physical activities. From where do you get this energy?
The digestion of the food we eat gives us energy.
How do plants get their food to grow?
All living things, including plants, require food to survive. Photosynthesis is the process they use to make their food. During photosynthesis, a plant's leaves absorb energy from sunlight. It also absorbs water from its roots as well as carbon dioxide from the air.
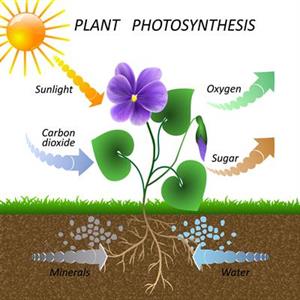
Photosynthesis
How does a car move using fuel?
The car moves due to the combustion of fuel. The potential chemical energy of gasoline is converted into kinetic energy at the wheels to drive a car. This is achieved through the combustion of gasoline, which results in gas expansion and air pollution.
Why does iron rust when it is exposed to water or air?
Rust is an iron oxide, a reddish-brown oxide that forms when iron and oxygen react (oxidation of iron) in the presence of water or air moisture.

Rusted iron
The above processes are examples of chemical changes, which means that the materials that change are converted into new materials.
Let us look at chemical reactions and their types in the upcoming sections.
Reference:
https://th.bing.com/th/id/OIP.5sA-hybhmA-BP-KJM7ZDigHaE8?w=240&h=180&c=7&o=5&pid=1.7
Reference: