
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoBased on the valence electrons present in an atom, the elements are classified as monovalent, divalent, trivalent, and so on.
Monovalent elements:
The elements that have a valency of one are called monovalent elements.
Example: Hydrogen and sodium
Divalent elements:
The elements that have a valency of two are called divalent elements.
Example: Oxygen and beryllium
Trivalent elements:
The elements that have a valency of three are called trivalent elements.
Example: Nitrogen and aluminium
Some elements exhibit more than one valency.
Example:
Iron combines with oxygen to form two types of ferrous oxide (exhibits valency 2) and ferric oxide (exhibits valency 3).
When atoms of different elements are combined, then the molecules of compounds are formed. During molecule formation, it is necessary to know the valancies of those elements.
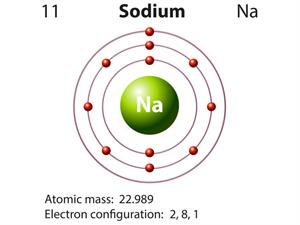 | 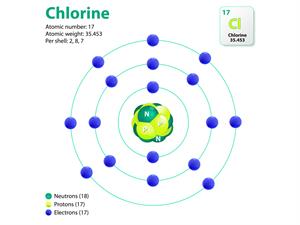 |
Atomic structure of sodium and chlorine
Here, the valancies of both sodium and chlorine are one. Therefore, the molecular formula will be NaCl.
But, when magnesium reacts with chlorine, it gives
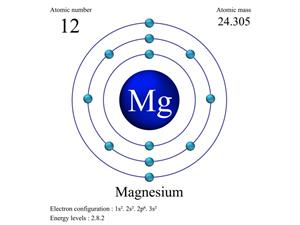 | 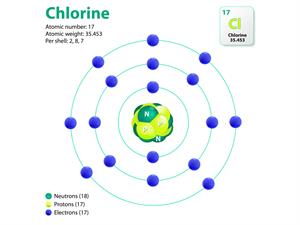 |
Atomic structure of magnesium and chlorine