
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoFreezing and condensation are exothermic processes as heat is removed, resulting in decreasing the molecules' speed, causing them to move slower.
The change that occurs in the state of a liquid into a solid on cooling is known as freezing.
States of matter
We know that solid has a strong force of attraction as the molecules are tightly packed. So when the liquid freezes, the particles arrange themselves as in the case of solid.
In liquids, the molecules are loosely packed with a weak intermolecular force, which is lower than solids.

Freezing
So when the temperature is lower, the molecules do not possess much energy to vibrate. Hence, the thermal or heat energy decreases, causing the liquid to shrink or contract, forming a solid from the liquid state. If the temperature is very low, freezing takes place at a faster rate.

Water freezes to form ice
Freezing is a physical change as the water and ice's chemical composition are the same except their state. This is an exothermic process.
Note: Water has the same melting and freezing point at .

Clouds
The supercooled liquids are ones in which the liquids are cooled at the temperature below their freezing point without turning into solid. For example, clouds at high altitudes are not solids but supercooled liquids.
Fact: The freezing and melting point of the pure substance are the same.
Condensation:
The process where the water vapour changes into the water on cooling is known as condensation.

Condensation during cooking
During cooking, we can see how the water vapour comes out and goes up. When the plate is covered during the cooking, the temperature goes down.
Fact: The condensation process is opposite to the evaporation process.
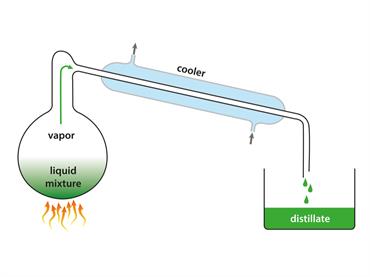
Vapours cool to form a liquid.
Condensation happens when molecules in a gas cool down.

Water cycle
In the water cycle, the clouds are formed from water vapour. The liquid obtained in condensation can be reverted into a gaseous state upon heating. The arrangement of molecules takes place from liquid to gas.

Formation of dew drops
Example:
Sweating of cold water bottles, fog, cloud formation, the change in temperature (from hotter to cooler), etc.
Both freezing and condensation is a physical change and an exothermic process as they release heat, causing the change in their state. This is an example of physical change as only the physical properties of the substance changes and not the chemical properties. Freezing and condensation are the two reactions that occur when the temperature is low.