PDF chapter test TRY NOW
We know that matter consists of tiny particles, atoms, and molecules.
States of matter
When the particles' arrangement in a substance changes by applying pressure, altering temperature, and for other different reasons, the physical state of the substance gets changed.
Characteristics of states of matter:
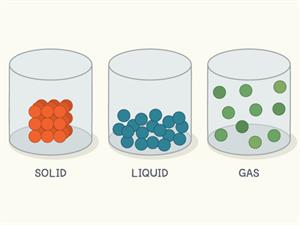
States of matter
Solid:
- In solid, the molecules are closely packed with no space to move.
- They have definite shape and volume.
- Due to their arrangement, they have a strong intermolecular force and less intermolecular space.
Liquid:
- In a liquid, the molecules are loosely packed.
- They do not have a definite shape but have a definite volume.
- Due to their arrangement, they have less intermolecular force and more intermolecular space compared to solids.
Gases:
- In gases, the molecules are very loosely packed.
- They do not have a definite shape and volume.
- Due to their arrangement, they have a very weak intermolecular force and a very large intermolecular space.
Let us see what happens when we apply heat to the substances:
On applying heat to these different states of matter, they all expand. When heat is added, the molecules gain energy and vibrate, and force other molecules to move apart. This expansion is minimum in solid compared with liquid and gases.
Expanding capacity: Gases<Liquid <Solid

Effect of heat on states of matter
Solid:
When heat is applied, they expand by a small amount.
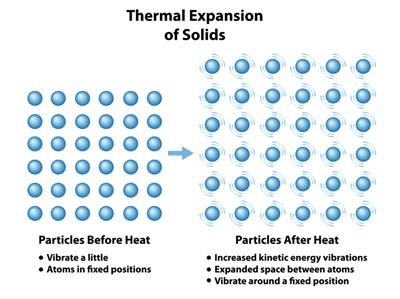
Effect of heat on solids
Reason: The particles are held by very strong intermolecular forces that start to vibrate faster at their fixed rate. But the force of attraction is stronger, which keep them close together. Therefore, only a small expansion occurs due to strong bonds present in solids.
Effect of heat on solid
Liquid:
When heat is applied, the liquid expands moderately.
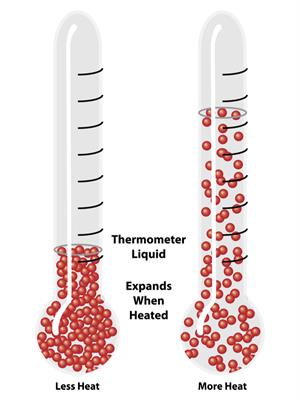
Expansion in liquids
Reason: Particles move around each other faster where the force of attraction between these particles is less than solids, which makes liquids expand more than solids.
Effect of heat on liquid
Gases:
When heat is applied, gases expand to a larger extent.
Effect of heat on gases
Reason: Particles are held very loosely, and so they move faster compared to liquids. They collide with a large amount of force with the walls of the container.
Effect of heat on gas
Note: On cooling, the different states of matter contract where their volume decreases. When a glass of water is heated, its volume increases; when it is cooled, the volume decreases. Such changes where there is a change in volume but mass remaining the same are called physical changes.
