PDF chapter test TRY NOW
Cell:
A cell is a device which converts chemical energy into electrical energy. It has two terminals; when these two terminals are shorted with a conducting wire, current will flow in the circuit.
Construction:
- An electrolyte is a chemical solution which produces positive and negative ions.
- Two different metal plates are inserted into an electrolyte as electrodes to form a cell.
- Due to chemical reactions, one electrode gets a positive charge, and the other receives a negative charge.
- When the ends of the electrodes are connected with any conducting material, then it produces a continuous flow of electric current.
Electrolyte
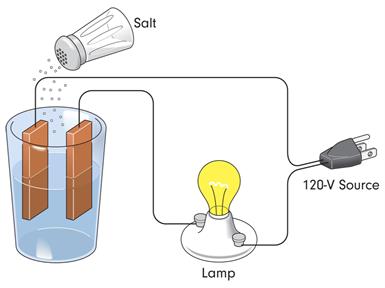
In the above diagram, brown plates act as electrodes
Depending on the continuity of flow of electric current, cells are classified into two types. They are
- Primary cells
- Secondary cells
Primary cells:
Primary cells can be used only once since they cannot be recharged again and again. Therefore, the primary cells are usually produced in small sizes.
Example:
Cells are used in clocks, watches and some toys.
| Cells used in wall clocks |  |
| Button cells (used in wristwatches) |  |
| Different types of cells used in toys |  |
Secondary cells:
- A cell which can be recharged many times is called a secondary cell, that can be recharged by passing an electric current. So secondary cells can be used again and again.
- The size of the secondary cells can be small or even large, depending upon the usage. Mobile phone consumes less charge, so the secondary cells used in mobiles are in the size of a palm, Whereas the cells used in automobiles like cars and buses are large and very heavy because it consumes heavy current.
- Secondary cells are used in many devices such as mobile phones, laptops, emergency lamps and vehicle batteries.

Batteries used in mobile phones

Batteries used in cars
Let us see the differences between a primary cell and a secondary cell:
Primary cell | Secondary cell |
| The chemical reaction that takes place inside the primary cell is irreversible. | The chemical reaction that takes place inside the secondary cell is reversible. |
| Primary cells cannot be recharged. | Secondary cells can be recharged. |
| Primary cells can be used in torches, toys and other electrical devices. | Secondary cells can be used in mobile phones, cameras, computers and emergency lights. |
Example: Simple voltaic cell, Daniel cell, Leclanche cell, and dry cell. | Example: Lead accumulator, Edison accumulator and Nickel-Iron accumulator. |
Reference:
https://upload.wikimedia.org/wikipedia/commons/8/81/01_-_Set_of_Energizer_Batteries.jpg
https://commons.wikimedia.org/wiki/File:Li-Ion_batteries_for_mobile_phones.jpg
https://commons.wikimedia.org/wiki/File:Start_Stop_Automotive_Battery.jpg
