PDF chapter test TRY NOW
The elements in the periodic table are classified as metals and non-metals based on their physical and chemical properties. There are around 95 metals and 17 non-metals in the periodic table.
Metals are electropositive elements, where they donate electrons to form a stable configuration.
Physical properties of metals:
A physical property can be observed and measured without altering the sample's chemical identity. In other words, a physical property can cause a physical change but not a chemical change.
Let's see some of the physical properties of metals.
- State
- Lustre
- Malleability
- Ductility
- Hardness
- Valency
- Conduction
- Density
- Sonorous
- Melting and Boiling Points
State:

Most of the metals are solid at room temperature, except mercury which is liquid at room temperature.
Lustre:

Metals are lustrous in nature (shining or the reflecting nature of metals), such as gold and silver.
Malleability:

Metals are malleable in nature (as they can be drawn into thin sheets).
Ductility:

Metals are ductile in nature (as they can be drawn into wires).
Hardness:

All metals are hard except sodium and potassium.
Valency:
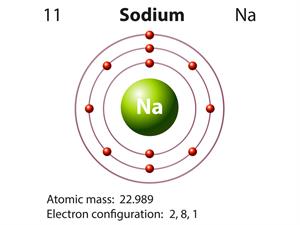
Metals have 1 to 3 electrons in the outermost shell.
Conduction:

Metals are good conductors of heat and electricity (they have free electrons in their outermost shell).
Density:

Metals have a high density (mass of unit volume of a material substance).
Sonorous:

Metals are sonorous in nature (they produce a ringing sound when struck hard).
Melting and Boiling Points:

Metals have high melting and boiling points because of their strong metallic bonds, except for sodium and potassium, which has a low melting and boiling points.
The materials which generally possess the above properties are called Metals.
Example:
Iron, copper, aluminium, calcium, magnesium, gold, silver etc.
