PDF chapter test TRY NOW
We will now see the difference between elements and compounds with the help of few examples.
Elements:

Elements cannot be further separated (gold, silver)
Substances that cannot be broken down to the simpler form of substances is known as the elements.
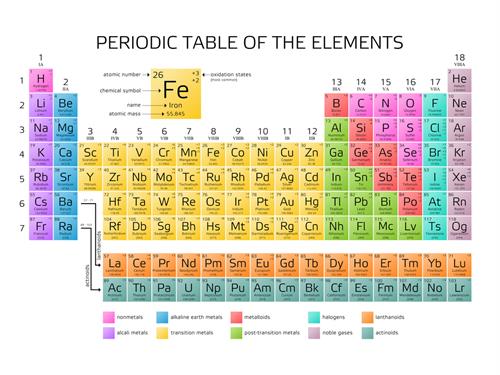
The elements are made of only one kind of atom and these are the fundamental particle.
Example:
Oxygen, nitrogen, helium, hydrogen.
Compounds:

Compounds can be separated
These substances have fixed components and can be broken down into elements by chemical reactions.
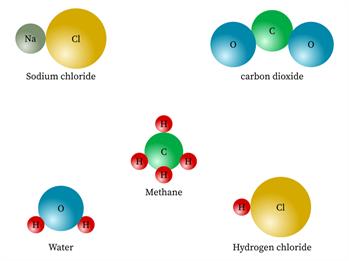
But the compounds are made of more than one kind of elements.
Example:
Sugar, water, salt, alcohol, baking soda.
Let's see some of the elements:
Pure Substance | Column I | Column II |
Argon Ar | Element | Cannot be separated |
Lead Pb | Element | Cannot be separated |
Phosphorous P_4 | Element | Cannot be separated |
Neon \(Ne)\ | Element | Cannot be separated |
Sulphur S_8 | Element | Cannot be separated |
Example:
Let's see some of the elements present in the compounds:
1. 

Chemical name: Water
Chemical formula: H_2O
Elements: Hydrogen, Oxygen.
Number of different elements: 2
2. 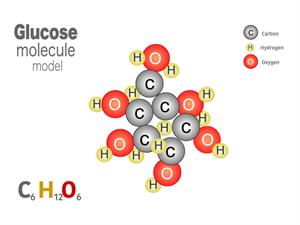

Chemical name: Glucose (Sugar)
Chemical formula: C_6H_{12}O_6
Elements: Carbon, Hydrogen, Oxygen.
Number of different elements: 3
3. 

Chemical name: Calcium oxide
Chemical formula: CaO
Elements: Carbon, Oxygen.
Number of different elements: 2
4. 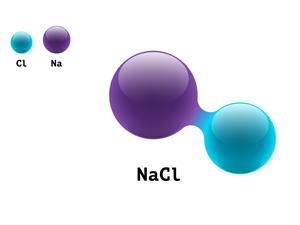

Chemical name: Sodium chloride (salt)
Chemical formula: NaCl
Elements: Sodium, Chlorine.
Number of different elements: 2
5. 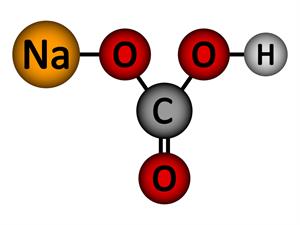

Chemical name: Sodium bicarbonate (Baking soda)
Chemical formula: NaHCO_3
Elements: Sodium, Carbon, Hydrogen, Oxygen.
Number of different elements: 4
6. 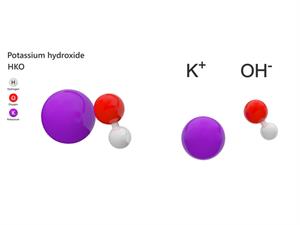

Chemical name: Potassium hydroxide
Chemical formula: KOH
Elements: Potassium, Oxygen, Hydrogen.
Number of different elements: 3
In the above examples, the chemical names such as water, glucose, calcium oxide, sodium chloride, sodium bicarbonate and potassium hydroxide are called compounds. These can be separated into their elements through chemical methods.
