PDF chapter test TRY NOW
We know that matter is made up of tiny particles; particles are constant and random. Lets us now see the difference between the states of matter. The following below are the 3 states of matter.
- Solid
- Liquid
- Gases
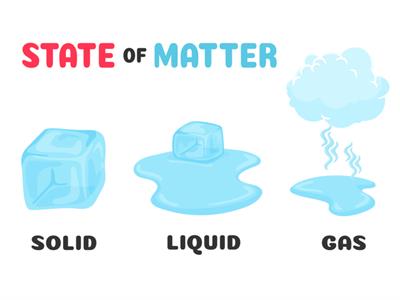
Characteristics of states of matter:
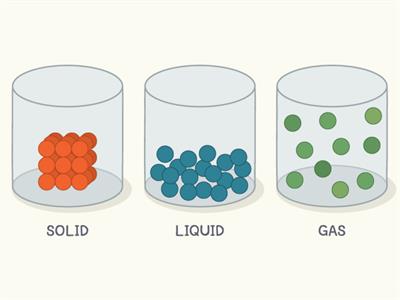
Packing in states of matter
Solid:

Solid
- In solid, the molecules are closely packed with no space to move.
- They have definite shape and volume.
- Due to their arrangement, it has a strong intermolecular force and less intermolecular space.
Liquid:

Liquid
- In liquid, the molecules are loosely packed.
- They don't have a definite shape but have a definite volume.
- Due to their arrangement, they have less intermolecular force and more intermolecular space compared to solids.
Gases:

Wind
- In gases, the molecules are very loosely packed.
- They don't have a definite shape and volume.
- Due to their arrangement, it has a very weak intermolecular force and a very large intermolecular space.
States of matter
Example:
Let us see how does hot-air balloon manage to stay in the air:

When a burner heats the air inside a hot-air balloon, it expands. The density of the air inside the balloon decreases as the balloon expands.
As a result, the density of the air inside the balloon is lower than that of the air outside the balloon.
The hot-air balloon will float because of the density difference.
