PDF chapter test TRY NOW
We know the difference between an atom, an element and a compound. Let us now learn about the term called 'atomicity'.
In chemistry, atomicity refers to the total number of atoms present in a single molecule of an element, compound, or substance. The number of atoms present in a single molecule is termed its atomicity.
We can distinguish the molecules respective to the number of elements they combined to form a molecule.
Let's look at how to figure out while calculating the atomicity of elements and compounds:
Let's look at how to figure out while calculating the atomicity of elements and compounds:
Example:
1. 

O + O \rightarrow O_2
Here, O refers to Oxygen atom.
Therefore, to calculate the atomicity, simply add the number of atoms together 1 + 1 \ = 2
Oxygen is a diatomic molecule, meaning that each molecule contains two atoms, giving it an atomicity of two.
2. 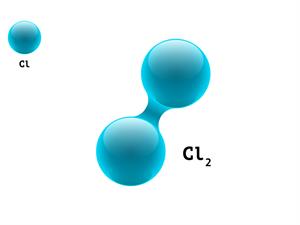

Cl + Cl \rightarrow Cl_2
Here, Cl refers to Chlorine atom.
1 + 1 \ = 2
Chlorine is a diatomic molecule. It means that each molecule contains two atoms, giving it an atomicity of two.
3. 

Na
Here, Na refers to sodium atom.
1 atom.
Sodium is a monoatomic element. It means that each molecule contains one atom, giving it atomicity of one.
For a molecule with multiple elements,
1. 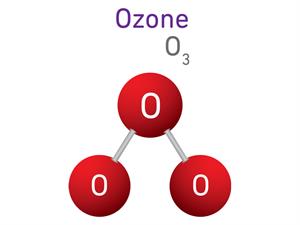

O_2 + O \rightarrow O_3
Here, O refers to Oxygen atom, and O_2 refers to oxygen molecule.
2 + 1 \ = 3
Ozone is a triatomic molecule, meaning that each molecule contains three atoms, giving it an atomicity of three.
2. 

C + O_2 \rightarrow CO_2
Here, C refers to Carbon atom, and O_2 refers to oxygen molecule.
1 + 2 \ = 3
Carbon dioxide is a triatomic compound, meaning that each molecule contains three atoms, giving it an atomicity of three.
3. 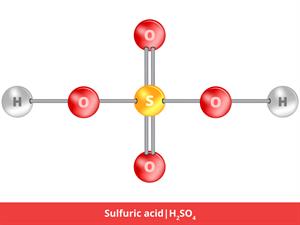

H_2SO_4
Here, H_2 refers to hydrogen, and O_4 refers to oxygen, and S refers to sulphur.
2 + 1 + 4 \ = 7
Sulphuric acid is a compound, giving it an atomicity of seven.
4. 

H_2O
Here, H_2 refers to hydrogen, and O refers to oxygen.
2 + 1 \ = 3
Water is a compound, giving it an atomicity of three.
5. 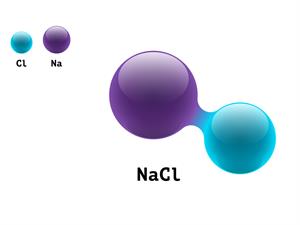

NaCl
Here, Na refers to sodium atom, and Cl refers to chlorine atom.
1 + 1 \ = 2
Sodium Chloride is a compound, giving it an atomicity of two.
Few more examples for elements and their atomicity.
Classification of elements | Name of the elements | Symbol | Atomicity |
Metals | Aluminum | Al | 1 |
Copper | Cu | 1 | |
Iron | Fe | 1 | |
Non-metals | Helium | He | 1 |
Hydrogen | H_2 | 2 | |
Nitrogen | N_2 | 2 | |
Fluorine | F_2 | 2 | |
Phosphorous | P_4 | 4 |
Reference:
