PDF chapter test TRY NOW
John Dalton:
1. John Dalton was born to a poor weaver's family in England in 1766. At the age of twelve, he started his career as a teacher. He became a school principal seven years later.

John Dalton
2. Dalton moved to Manchester in 1793 to teach mathematics, physics, and chemistry at university.
3. He taught and did study there for the majority of his time. He proposed his atomic theory in 1808, which was a watershed moment in the study of matter.
4. John Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
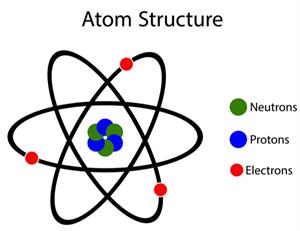
According to John Dalton, all matters, whether an element, a compound or a mixture, are composed of tiny particles called atoms.
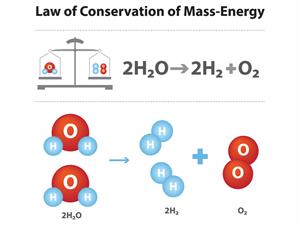

Atoms can neither be created nor destroyed.
The following postulates are proposed by John Dalton:
- Atoms are the tiniest particles that comprise all matters.
- In a chemical reaction, atoms are indivisible particles that cannot be formed or destroyed.
- A given element's atoms have the same mass and chemical properties.
- The masses and chemical properties of atoms in various elements vary.
- Compounds are formed when atoms combine in small whole-number ratios.
- In a given compound, the relative number and type of atoms remain constant.
Dalton's atomic theory
We got an idea about an atom from Dalton's atomic theory, but first of all, we should know what an atom is. In the next lesson, we will explore more about the atom.
