
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoValency in terms of hydrogen, oxygen and chlorine:
The valency of an element is also measured concerning other atoms, like hydrogen (H), oxygen (\(O_2\)) and chlorine (Cl).
Valency about hydrogen:
Since the hydrogen atom loses one electron in its outermost orbit, its valency is assumed to be one, as chosen by the norm. Therefore, valencies of the other elements are shown in terms of hydrogen. Hence, the number of hydrogen atoms that join with one atom of an element is known as its valency.
Example:
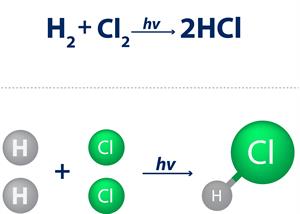
Hydrogen valency
In a hydrogen chloride molecule, one hydrogen atom joins with one chlorine atom. Hence,
the valency of chlorine is one.
the valency of chlorine is one.
Example:
Since certain elements don't mix well with hydrogen, the element's valency is also expressed in terms of other elements, such as chlorine or oxygen. It is because almost every element reacts with chlorine and oxygen.
Valency of atoms:
Molecule | Element | Valency |
| Hydrogen chloride (HCl) | Chlorine | 1 |
| Water (H_2O) | Oxygen | 2 |
| Ammonia (NH_3) | Nitrogen | 3 |
| Methane (CH_4) | Carbon | 4 |