PDF chapter test TRY NOW
Watermelon Model:
- In \(1904\), J.J.Thomson proposed an atom model after the discovery of electrons.

- Thomson suggested that the shape of an atom relates to a sphere (radius m).
- The positively charged particles are evenly distributed with electrons arranged so that the atom is electrically neutral.

- The name of Thomson's atom model is the Plum pudding model or Watermelon model. The settled electrons are related to the seed of the watermelon.
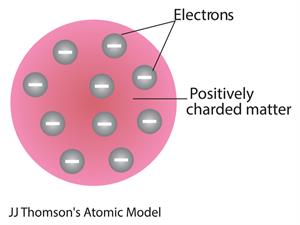
- Watermelon’s red mass expressed the positive charge distribution.
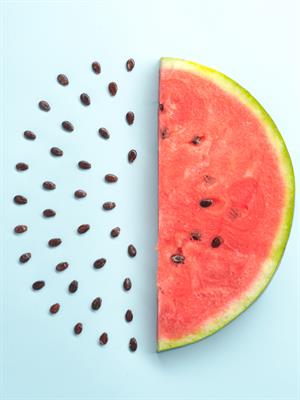
Watermelon modal
- The Plum pudding theory concluded that 'the mass of an atom is evenly spread all over the atom'.
Note: Thomson’s model successfully explains the electrical neutrality of the atom.
Limitations of Thomson’s Model:
- Thomson's atomic model could not explain the positive charge on the electrons inside the atom.
- This model explains only for protons and electrons, not for neutron and nucleus.
- It did not explain the stability of an atom.
- It failed to explain Rutherford's scattering experiment.
