PDF chapter test TRY NOW
Proust's law:
In \(1779\), Joseph Proust proposed a law of constant proportions. According to the statement,
The elements are always contained in definite proportions by mass in a pure chemical compound.
He looked at all the compounds of two or more elements and found that they all possess the same elements in the same proportions, regardless of where they came from or who made them.
Example:
Water from various sources, such as rain, well, sea, and river, will always contain the same two elements, oxygen and hydrogen, in the ratio of 1:8 by mass.
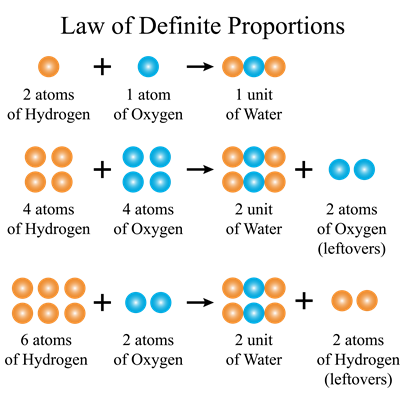
Similarly, while the method of preparation of compounds may differ, their composition will never change. It will be in a permanent ratio. As a result, this law is also known as the 'Law of definite proportions.'
