PDF chapter test TRY NOW
Do you imagine a life without electricity?

Certainly not. Electricity is essential for our survival. Cooking, lighting, grinding, watching TV, charging cell phones, laptops, computers, and water heaters are few examples of how we use electricity.

You will be surprised to know that electricity can be used to carry out chemical reactions.
Many chemical reactions are carried out with the help of electricity, which is very significant in the industrial world.
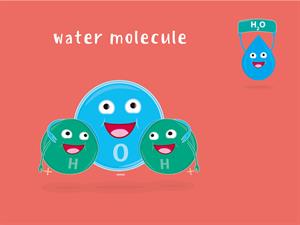
Water is made up of hydrogen and oxygen ions, as you already know. Hydrogen and oxygen gases are liberated as electricity is passed through water containing small quantities of sulphuric acid.
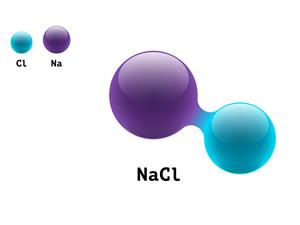
In a similar way, brine, a concentrated sodium chloride solution, is electrolysed to obtain chlorine and hydrogen gases as well as sodium hydroxide. This is a key reaction in the industrial production of chlorine.
We can observe from the above two reactions that certain chemical reactions require only the passage of electricity. As a result, these reactions are referred to as electrochemical reactions or electrolysis.
Note: Michael Faraday coined the word electrolysis in the 19th century. Electrolysis is a term that combines both electron and lysis. The electron is associated with electricity, and lysis refers to the process of decomposition.
