PDF chapter test TRY NOW
The matter is made up of small particles. These small particles are known as atoms and molecules that can transfer heat. The atoms and molecules have a motion at any time. These motions are responsible for the energy that they possess. The more the motion indicates the substance has higher energy (thermal or heat). However, heat transfer is nothing but the transfer of heat from a high-temperature object to a low-temperature object.
What is heat transfer?
Heat transfer is defined as the transfer of heat across the boundary of the system because of the difference in temperature between the system and its surroundings.
How is heat transferred?
If heat energy is supplied to any substance, it will be transferred from one part of the substance to another. It takes place in different ways, depending on the state of the substance.
When there are objects at different temperatures, or there is an object at a different temperature from the surroundings, then the transfer of heat takes place so that the object and the surrounding reach thermal equilibrium (same temperature).
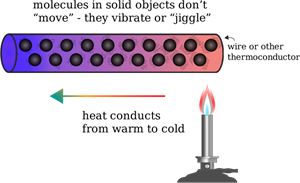
- Conduction
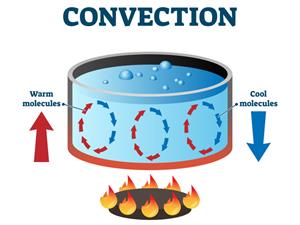
- Convection
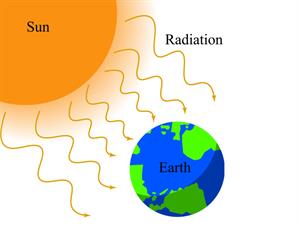
- Radiation
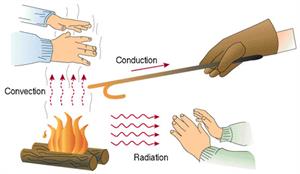
Thermal Equilibrium:
Two systems are said to be in thermal equilibrium if there is no transfer of heat between them, and both have the same temperature.
Reference:
https://commons.wikimedia.org/wiki/File:Heat-transmittance-means1.jpg
