PDF chapter test TRY NOW
A calorimeter is an instrument is used to measure the amount of heat gained or lost by a substance. It contains a vessel made up of metals like copper or aluminium, good conductors of heat and electricity.
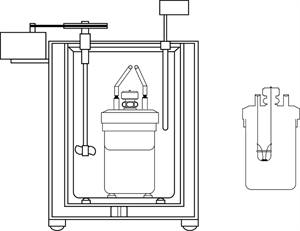
Calorimeter
The metallic vessel is placed in an insulating jacket to avoid heat loss to the surroundings (environment). There are two holes in the vessel. A thermometer is inserted through one hole to measure the temperature of the materials. Through another hole, a stirrer is inserted to stir the content inside the vessel. The liquid filled inside the vessel is heated by passing a current through the heating element. Using this instrument, we can measure the heat capacity of the liquid in the container.
Working:
In a calorimeter, two forms of matter (generally solids and liquids) with different temperatures are made to be in physical contact with one another. Due to this temperature difference, the heat is transferred from higher temperature to lower temperature. This process of heat transfer continues until the thermal equilibrium reaches.
The principle of calorimetry indicates the law of conservation energy. It means that the total heat lost by the hot body is equal to the total heat gained by the cold body.
Heat lost from the hotter body = Heat gained by the colder body
The amount of heat transfer is calculated by the formula,
The unit of heat transfer is Joule.
