PDF chapter test TRY NOW
Isomerism: Isomerism is another unique feature of carbon compounds mainly observed in catenated organic compounds. For example, let us think of the molecular formula of an organic compound (\(C_2H_6O\)).
1. Can you name the compound? Unfortunately, you cannot, because the molecular formula of an organic compound describes only the number of various atoms present in that compound.
2. It does not tell about how the atoms are organised and hence their structure. Without understanding the structure, we cannot name the compound.
This phenomenon in which the compound has same molecular formula but a different structural arrangement is called isomerism.
Compounds with the same molecular formula but different structural formulas are called isomers.
A provided molecular formula may lead to more than one form of atoms. In addition, such compounds possess different physical and chemical properties.
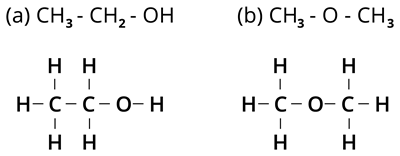
The given formula (\(C_2H_6O\)) has two kinds of arrangement of atoms, as shown below.
Isomerism
Both the compounds possess the same molecular formula but different kinds of arrangements.
In compound (\a\), the oxygen atom is attached to hydrogen and carbon, called Alcohol.
In compound (\b\), the oxygen atom is attached to two carbon atoms, which is Ether.
These compounds have various physical and chemical characteristics. You will study more about isomerism in your higher classes. Allotropy:
Allotropy is a quality by which an element can exist in more than one physically different and chemically similar form. Thus, the various forms of that element are called its Allotropes.
The main reason for the existence of allotropes of an element is its method of formation or preparation.
Example:
Carbon exists in different allotropic forms. Based on their physical nature they are classified as below.
(a). Crystalline forms of Carbon:
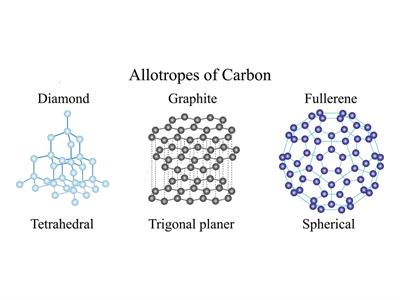
Diamond:
- Each carbon atom gives its four valence electrons, with four other carbon atoms forming four covalent bonds in a diamond.
- Here, the atoms are arranged in a repeated tetrahedral fashion, leading to a three-dimensional structure accounting for its hardness and rigidity.
Graphite:
- Every carbon atom in graphite is covalently connected to three other carbon atoms in the same plane.
- This arrangement forms hexagonal layers, which are held together with one other by weak Vander Waals forces.
- Since weak forces have layers, Graphite is softer than diamond.
Difference between Diamond and Graphite:
Diamond | Graphite |
| Each carbon has four covalent bonds. | Each carbon has three covalent bonds. |
| Hard, heavy and transparent. | Soft, slippery to touch and opaque. |
| It has tetrahedral units linked in three dimensions. | It has planar layers of hexagon units. |
| It is a non-conductor of heat and electricity. | It is a conductor of heat and electricity. |
Fullerene: Fullerene is the third crystalline allotrope of carbon.
The best-known fullerene is Buckminsterfullerene, consisting of \(60\) carbon atoms joined together in five and six-membered to form a spherical molecule resembling a soccer ball. So its formula is \(C60\).
1. This allotrope was described Buckminsterfullerene after the American architect Buckminster fuller. However, because Fuller's structure reminded the framework of dome-shaped halls for great international expositions, Bucky Ball is its pet name.
2. Fullerenes come in a wide range of sizes, starting at \(C20\) and up to \(C540\).
Fullerenes
(b). Amorphous forms of carbon: In an amorphous form of carbon, carbon atoms are arranged randomly. These forms of carbon are obtained when the wood is heated in the absence of air. E.g., charcoal.

Charcoal
