
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free Demo Sodium chloride (NaCl):
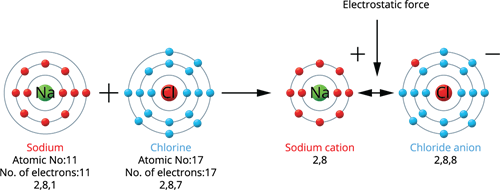
Sodium holds an atomic number of 11 and an electronic configuration of 2, 8, 1. Thus, it has one electron more than the nearest stable noble gas electronic configuration, Neon. As a result, sodium tends to lose one electron from its outermost shell and obtain a stable electronic configuration, resulting in a sodium cation (Na^+).
Ionic bond formation in sodium chloride
Chlorine holds an atomic number of 17 and an electronic configuration of 2, 8, 7. It has one lower electron than the closest stable noble gas electrical configuration - Argon. As a result, chlorine tends to gain one electron to achieve a stable electronic configuration, resulting in the formation of the chloride anion (Cl^-).
Ionic bond formation in sodium chlorine
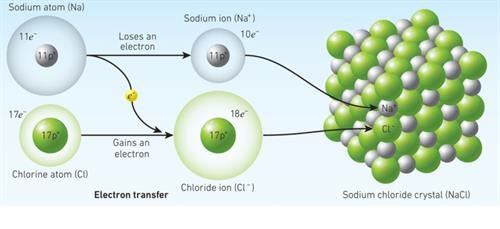
When a sodium atom joins with a chlorine atom, one electron is transferred from the sodium atom to the chlorine atom, resulting in a sodium chloride molecule.Thus, both atoms have a stable octet electronic configuration.
Magnesium chloride(MgCl_2):
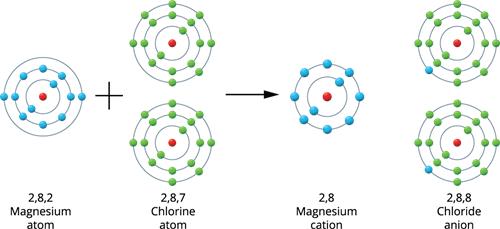
Magnesium holds an atomic number of 12, and its electronic configuration is 2, 8, 2. Thus, it has two more electrons than the closest stable electronic configuration of a noble gas, Neon. As a result, magnesium tends to lose two electrons from its outermost shell and obtains a stable electronic configuration, resulting in magnesium cation (Mg^2{^+}).
Ionic bond formation in Magnesium chloride
When a magnesium atom joins two chlorine atoms, two electrons are transferred from the magnesium to the chlorine, resulting in a magnesium chloride molecule. Thus, both atoms have a stable octet electronic configuration.