
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe experiment requires copper sulphate solution, carbon rod, copper wires, and dry cell or battery.
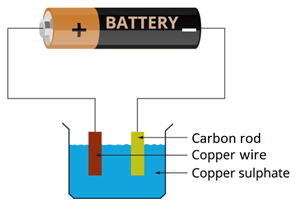
Experimental set-up
Procedure:
- Fill a beaker halfway with copper sulphate solution.
- Take a carbon rod from an old dry cell or battery.
- Wind a wire on the upper end of a carbon rod.
- Clean a thick copper wire and flatten it with a hammer.
- Immerse the copper wire and carbon rod in the copper sulphate solution at the same time.
- Connect the negative terminal of an electric cell to the carbon rod and the positive terminal to the copper wire.
- Place the copper wire and the carbon rod close to each other but ensure that it does not make any contact.
- Now, pass the current for some time.
Observation:
After some time, fine copper will get deposited over the carbon rod.
After some time, fine copper will get deposited over the carbon rod.
Inference:
When we pass the current through the copper sulphate solution, it dissociates into copper and sulphate. The copper ions are then deposited into the carbon rod connected to the negative terminal of the battery. The copper plate helps restore the copper loss in the solution. This restoration gives an equal amount of copper to the solution, and the process continues. Finally, the copper gets completely transferred to the carbon rod through the solution.
When we pass the current through the copper sulphate solution, it dissociates into copper and sulphate. The copper ions are then deposited into the carbon rod connected to the negative terminal of the battery. The copper plate helps restore the copper loss in the solution. This restoration gives an equal amount of copper to the solution, and the process continues. Finally, the copper gets completely transferred to the carbon rod through the solution.
So far, we have only encountered situations where just electrons can conduct electricity. But, both the electron and the positive copper ion conducts electricity when current flows through an electrolyte (Copper sulphate solution).
The process of conduction of electric current through solutions is called Electrolysis. The solution through which the electricity passes is called an Electrolyte.
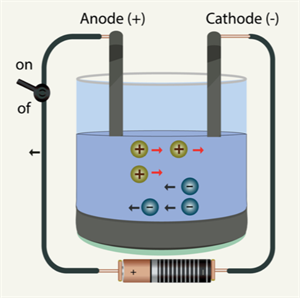
Electrolysis
The anode is the positive terminal inserted into the solution, and the cathode is the negative terminal. In the above case, the copper wire is the anode, and the carbon rod is the cathode.
Important!
Synaptic signals are extremely weak electric currents produced in the human body by the movement of charged particles. An electrochemical process produces these signals, and these signals travel between the brain and the organs through the nervous system.
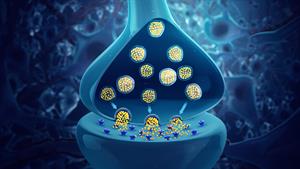
Synaptic signals

Synaptic signals
Magnetic effect of current:
In 1820, Hans Christian Oersted, a Danish physicist, conducted many experiments and proved the magnetic effect of electric current.A current-carrying conductor or a wire develops a magnetic field perpendicular to the direction of the flow of current. This is known as the Magnetic effect of current.
Right-hand thumb rule:
A wire is held with four fingers in the right hand with a thumbs-up position. Here, the thumb of the right hand indicates the direction of the current (I) whereas the other fingers of the same hand indicate the direction of the magnetic field (B).
A wire is held with four fingers in the right hand with a thumbs-up position. Here, the thumb of the right hand indicates the direction of the current (I) whereas the other fingers of the same hand indicate the direction of the magnetic field (B).
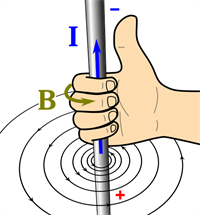
Right-hand thumb rule
Reference:
https://upload.wikimedia.org/wikipedia/commons/0/01/Neurotransmitters.jpg