PDF chapter test TRY NOW
- Take a beaker half filled with copper sulphate solution.
- Take a carbon rod from a used dry cell.
- Wind a wire on its upper end.
- Take a thick copper wire, clean it well and flatten it with a hammer.
- Immerse both the copper wire and carbon rod in the copper sulphate solution.
- Connect the carbon rod to the negative terminal of an electric cell and copper wire to the positive terminal of the cell.
- Also ensure that the copper and the carbon rod do not touch each other, but are close enough.
- Wait and watch.
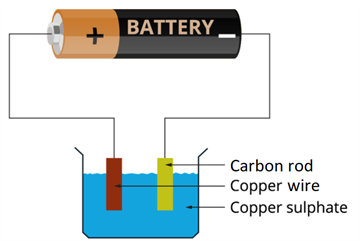
Experimental set-up
After some time you would find deposited over the . This is called as . This is due to the .
Inference:
When the current is passed through the , it dissociates into . The copper ions are then deposited into the carbon rod connected to the of the battery.
The copper plate helps restore the in the solution. This restoration gives an equal amount of copper to the solution, and the process continues. Finally, the copper gets completely transferred to the carbon rod through the .
The process of conduction of electric current through solutions is called . The solution through which the electricity passes is called an .
