
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe temperature at which the pressure and volume of a gas theoretically reach zero is called absolute zero.
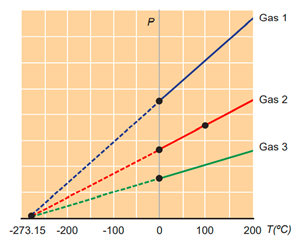
Variation of pressure with temperature
At a temperature of –273.15 °C, the pressure extrapolates to zero for all gases. It is called n as absolute zero or 0 K. Some baseline temperatures in the three temperature scales are shown below.
Temperature | Kelvins (K) | Degree celcius (°C) | Degrees Fahrenheit (°F) |
| Boiling point of water | 373.1 | 100 | 212 |
| Melting point of ice | 273.1 | 0 | 32 |
| Absolute zero | 0 | -273 | 460 |
We can convert the values from one unit to another unit by using the formula,
a) Convert 30°C to Kelvin
Solution:
b) Convert 240 K to °C