
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe process of changing a substance from one physical state to another at a definite temperature is known as a change of state.
At room temperature, for example, water molecules are in a liquid state. When water is heated to \(100\) degrees Celsius, it turns into steam, a gaseous state of matter. When the temperature of the steam is reduced, it reverts to water. If we lower the temperature to \(0°C\), it turns into ice, which is a solid-state of water. When ice melts, it reverts to water. As a result, when the temperature changes, water changes its state. There are various such processes in the matter's change of state.
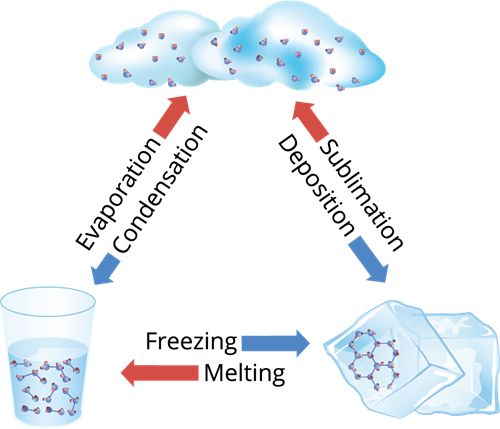
Change of state of matter
Melting-Freezing:
Melting or fusion is the process of converting a solid into a liquid by absorbing heat. The melting point is the temperature at which a solid transforms into a liquid. Freezing is the polar opposite of melting. Freezing is the process of converting a liquid to a solid by releasing heat. The freezing point is the temperature at which a liquid transforms into a solid. Melting and boiling occur at \(0°C\) in the case of water.
Boiling-Condensation: The temperature at which a liquid changes its state to gas is called the boiling point. The process in which vapour is converted to a liquid by releasing heat is called condensation. Condensation point is the temperature at which a gas changes from a gas to a liquid. Water has a boiling point of \(100°C\) and a condensation point of \(100°C\).
The process in which a liquid is converted to vapour by absorbing heat is called boiling or vaporization.
Sublimation:
Dry ice, iodine, frozen carbon dioxide, and naphthalene balls are examples of solids that change from solid to the gaseous state without becoming liquid.
Sublimation is the process of transforming a solid into a gaseous state.
Various stages of heat-induced state conversion with corresponding temperature changes.