PDF chapter test TRY NOW
Pure substances are those that are completely made up of one form of particle. Physical processes cannot distinguish pure substances from other forms of matter.
Example:
Salt, sugar, oxygen, copper, iron etc.,
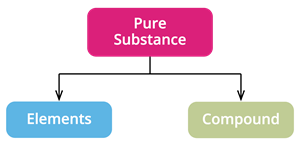
Based on the kind of atom, we classify pure substances as
- Elements and
- Compounds
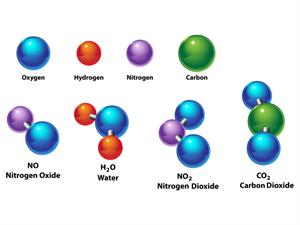
Compound is a form of matter created by combining two or more elements in a specific mass ratio. To decompose a compound into its constituent components chemical methods are used.
WaterH_2O, Oxygen O_2, Nitrogen dioxide NO_2, Salt NaCl, and so on.
WaterH_2O, Oxygen O_2, Nitrogen dioxide NO_2, Salt NaCl, and so on.
Elements are the fundamental substances that cannot be separated into simpler substances by any chemical methods. All the elements in the periodic table comes under this category such as helium, hydrogen, oxygen, iron, etc.
In 1661, Robert Boyle became the first scientist to use the term element.
Robert Boyle
A French chemist named Antoine Laurent Lavoisier 1743 - 1794 was the first to define an experimentally useful description of an element.

Antoine Laurent Lavoisier
An element, according to him, is a fundamental type of matter that cannot be broken down into simpler substances by chemical reactions.
- Metals
- Non-metals
- Metalloids
Let us now identify some of the elements and compound of a pure substance:
Pure Substance | Element /Compound | Reason why it is compound or element |
| Water H_2O | Compound | Can be separated as Water = Hydrogen + Oxygen |
| Lead Pb | Element | Cannot be separated |
| Phosphorous P_4 | Element | Cannot be separated |
| Sodium Chloride NaCl | Compound | Can be separated as Sodium Chloride = Sodium + Chloride |
| Sulphur S_8 | Element | Cannot be separated |
