
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLet us try to name some of the other classes of organic compounds using IUPAC rules:
1. 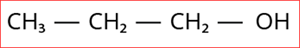
Step 1: According to rule 1, there is a three-membered carbon chain; hence, the root word is 'Prop'.
Step 2: According to rule 2, all the bonds between carbon atoms are single bond, and thus the primary suffix is ‘ane’.
Step 3: According to rule 3, the carbon chain is numbered from the end closest to the -OH group that has the lowest locant number, as shown below
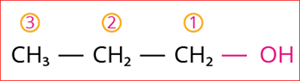
Numbering of the carbon chain
Step 4: The locant number is 1 for the -OH group, and hence the secondary suffix is ‘-1-ol’.
Note: According to rule 5, when the primary and secondary suffixes are combined, the primary suffix's terminal 'e' is removed.
Hence, the name of the given compound is \text{Prop + ane + (-1-ol) = Propan-1-ol}.
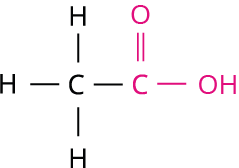
2. 

Step 1: According to rule 1, there is a two-membered carbon chain; hence, the root word is 'Eth'.
Step 2: According to rule 2, all the bonds between carbon atoms are single bonds, and thus the primary suffix is ‘ane’.
Step 3: The given compound contains carboxylic acid group (-COOH), and hence the secondary suffix is 'oic acid'.
Note: According to rule 5, when the primary and secondary suffixes are combined, the primary suffix's terminal 'e' is removed.
Hence, the name of the given compound is \text{Eth + ane + oic acid = Ethanoic acid}.
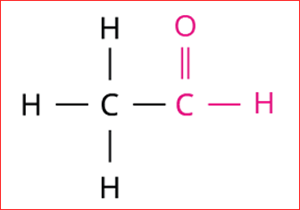
3. 

Step 1: According to rule 1, there is a two-membered carbon chain; hence, the root word is 'Eth'.
Step 2: According to rule 2, all the bonds between carbon atoms are single bond, and thus the primary suffix is ‘ane’.
Step 3: The given compound contains an aldehyde group (-CHO), and hence the secondary suffix is 'al'.
Note: According to rule 5, when the primary and secondary suffixes are combined, the primary suffix's terminal 'e' is removed.
Hence, the name of the given compound is \text{Eth + ane + al = Ethanal}.
4. 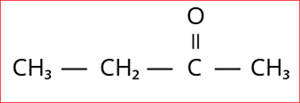
Step 1: According to rule 1, there is a four-membered carbon chain; hence, the root word is 'But'.
Step 2: According to rule 2, all the bonds between carbon atoms are single bonds, and thus the primary suffix is ‘ane’.
Step 3: The given compound contains ketone group (-C=O), and hence the secondary suffix is 'one'.
Step 4: The numbering of carbon chain occurs in such a way that the lowest possible number is given to the functional group in the chain.
So, its locant number is 2. Thus, the suffix is ‘2-one’.
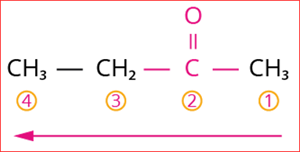
Numbering of the carbon chain
Note: According to rule 5, when the primary and secondary suffixes are combined, the primary suffix's terminal 'e' is removed.
Hence, the name of the given compound is \text{But + ane + (-2-one) = Butan-2-one}.
5. 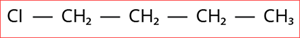
Step 1: According to rule 1, there is a four-membered carbon chain; hence, the root word is 'But'.
Step 2: According to rule 2, all the bonds between carbon atoms are single bonds, and thus the primary suffix is ‘ane’.
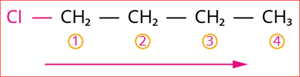
Step 3: The given compound contains halogen group (Cl), and hence the prefix is 'chloro'.
Step 4: According to rule 6, the substituent is a halogen group compound located at the first carbon atom.
The numbering of carbon chain occurs in such a way that the lowest possible number is given to the functional group in the chain.
Numbering of the carbon chain
So, its locant number is 1. Thus, the prefix is ‘1-chloro’.
Hence, the name of the given compound is \text{1-chloro + But + ane = 1-Chloro butane}.
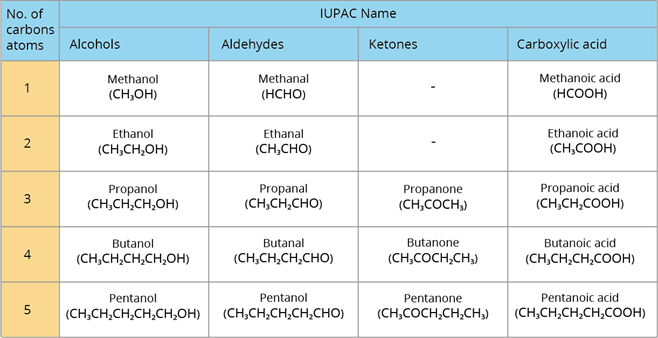
The below are some of the IUPAC names of various classes of compound:
In the upcoming section, we delve into the chemical properties of carbon compounds. It will explore the combustion of carbon and its compounds, particularly relevant due to their widespread use as fuels.