PDF chapter test TRY NOW
Let us try to name some of the linear and substituted hydrocarbons in a systematic manner using IUPAC rules:
1. CH_3-CH_2-CH_2-CH_2-CH_3
Step 1: According to rule 1, there is a five-membered carbon chain; hence, the root word is ‘Pent’.
Step 2: According to rule 2, all the bonds between carbon atoms are single bonds, and thus the suffix is ‘ane’.
So, the name of the given compound is \text{Pent + ane = Pentane}.
2. 

Step 1: According to rule 1, there is a five-membered carbon chain; hence, the root word is ‘Pent’.
Step 2: According to rule 2, there is a substituent. So, the carbon chain is numbered from the left end, which is closest to the substituent.

Step 3: All are single bonds between the carbon atoms, and thus the suffix is ‘ane’.
Step 4: According to rule 6, the substituent is a methyl group compound located at the second carbon atom.
So, its locant number is 2. Thus, the prefix is ‘2-Methyl’.
Hence, the name of the given compound is \text{2-Methyl + pent + ane = 2-Methyl pentane}.
3. 

Step 1: According to rule 1, there is a seven-membered carbon chain; hence, the root word is ‘Hept’. Step 2: According to rule 2, there is a substituent. So, the carbon chain is numbered from the left end, which is closest to the substituent.
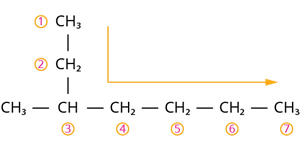
The correct way of numbering the carbon atoms

The incorrect way of numbering the carbon atoms
Step 3: All are single bonds between the carbon atoms, and thus the suffix is ‘ane’.
Step 4: According to rule 6, the substituent is a methyl group compound located at the third carbon atom.
So, its locant number is 3. Thus, the prefix is ‘3-Methyl’.
Hence, the name of the given compound is \text{3-Methyl + hept + ane = 3-Methyl heptane}.
4. 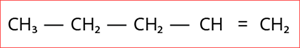
Step 1: According to rule 1, there is a five-membered carbon chain; hence, the root word is ‘Pent’.
Step 2: According to rule 2, there is a carbon to carbon double bond (C=C), and thus the suffix is ‘ene’.
Step 3: According to rule 3, the carbon chain is numbered from the end of the double bond that has the lowest locant number, as shown below
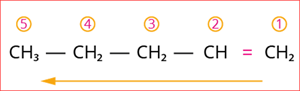
Numbering starts from the double bond.
Step 4: The locant number is 1 for the double bond, and hence the suffix is ‘-1-ene’.
Hence, the name of the given compound is \text{Pent + (-1-ene) = Pent-1-ene}.
These are some of the examples for naming the hydrocarbons according to the IUPAC rule. In the next section, we will see the IUPAC naming for various other compounds.
